Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli
- PMID: 18765794
- PMCID: PMC2532929
- DOI: 10.1101/gad.475808
Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli
Abstract
During the transition from post-exponential to stationary phase, Escherichia coli changes from the motile-planktonic to the adhesive-sedentary "lifestyle." We demonstrate this transition to be controlled by mutual inhibition of the FlhDC/motility and sigma(S)/adhesion control cascades at two distinct hierarchical levels. At the top level, motility gene expression and the general stress response are inversely coordinated by sigma(70)/sigma(FliA)/sigma(S) competition for core RNA polymerase and the FlhDC-controlled FliZ protein acting as a sigma(S) inhibitor. At a lower level, the signaling molecule bis-(3'-5')-cyclic-diguanosine monophosphate (c-di-GMP) reduces flagellar activity and stimulates transcription of csgD, which encodes an essential activator of adhesive curli fimbriae expression. This c-di-GMP is antagonistically controlled by sigma(S)-regulated GGDEF proteins (mainly YegE) and YhjH, an EAL protein and c-di-GMP phosphodiesterase under FlhDC/FliA control. The switch from motility-based foraging to the general stress response and curli expression requires sigma(S)-modulated down-regulation of expression of the flagellar regulatory cascade as well as proteolysis of the flagellar master regulator FlhDC. Control of YhjH by FlhDC and of YegE by sigma(S) produces a fine-tuned checkpoint system that "unlocks" curli expression only after down-regulation of flagellar gene expression. In summary, these data reveal the logic and sequence of molecular events underlying the motile-to-adhesive "lifestyle" switch in E. coli.
Figures


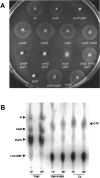

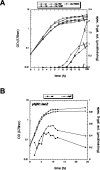


References
-
- Adler J., Templeton B. The effect of environmental conditions on the motility of Escherichia coli. J. Gen. Microbiol. 1967;46:175–184. - PubMed
-
- Barembruch C., Hengge R. Cellular levels and activity of the flagellar σ factor FliA of Escherichia coli are controlled by FlgM-modulated proteolysis. Mol. Microbiol. 2007;65:76–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
