Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity
- PMID: 18765800
- PMCID: PMC2533206
- DOI: 10.1073/pnas.0805706105
Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity
Abstract
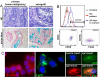
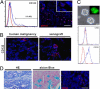
Colon carcinoma is one of the leading causes of death from cancer and is characterized by a heterogenic pool of cells with distinct differentiation patterns. Recently, it was reported that a population of undifferentiated cells from a primary tumor, so-called cancer stem cells (CSC), can reconstitute the original tumor on xenotransplantation. Here, we show that spheroid cultures of these colon CSCs contain expression of CD133, CD166, CD44, CD29, CD24, Lgr5, and nuclear beta-catenin, which have all been suggested to mark the (cancer) stem cell population. More importantly, by using these spheroid cultures or freshly isolated tumor cells from multiple colon carcinomas, we now provide compelling evidence to indicate that the capacity to propagate a tumor with all differentiated progeny resides in a single CSC. Single-cell-cloned CSCs can form an adenocarcinoma on xenotransplantation but do not generate the stroma within these tumors. Moreover, they can self-renew and are capable of multilineage differentiation. Further analysis indicated that the lineage decision is dictated by phosphoinositide 3-kinase (PI3K) signaling in CSCs. These data support the hypothesis that tumor hierarchy can be traced back to a single CSC that contains multilineage differentiation capacity, and provides clues to the regulation of differentiation in colon cancers in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: Models and concepts. Annu Rev Med. 2007;58:267–284. - PubMed
-
- Vermeulen L, et al. Cancer stem cells: Old concepts, new insights. Cell Death Differ. 2008;15:947–958. - PubMed
-
- Clarke MF, et al. Cancer stem cells: Perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. - PubMed
-
- O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. - PubMed
-
- Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

