Development of tRNA synthetases and connection to genetic code and disease
- PMID: 18765819
- PMCID: PMC2548368
- DOI: 10.1110/ps.037242.108
Development of tRNA synthetases and connection to genetic code and disease
Abstract
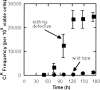
The genetic code is established by the aminoacylation reactions of aminoacyl tRNA synthetases, where amino acids are matched with triplet anticodons imbedded in the cognate tRNAs. The code established in this way is so robust that it gave birth to the entire tree of life. The tRNA synthetases are organized into two classes, based on their active site architectures. The details of this organization, and other considerations, suggest how the synthetases evolved by gene duplications, and how early proteins may have been statistical in nature, that is, products of a primitive code where one of several similar amino acids was used at a specific position in a polypeptide. The emergence of polypeptides with unique, defined sequences--true chemical entities--required extraordinary specificity of the aminoacylation reaction. This high specificity was achieved by editing activities that clear errors of aminoacylation and thereby prevent mistranslation. Defects in editing activities can be lethal and lead to pathologies in mammalian cells in culture. Even a mild defect in editing is casually associated with neurological disease in the mouse. Defects in editing are also mutagenic in an aging organism and suggest how mistranslation can lead to mutations that are fixed in the genome. Thus, clearance of mischarged tRNAs by the editing activities of tRNA synthetases was essential for development of the tree of life and has a role in the etiology of diseases that is just now being understood.
Figures


References
-
- An, S., Musier-Forsyth, K. Trans-editing of Cys-tRNAPro by Haemophilus influenzae YbaK protein. J. Biol. Chem. 2004;279:42359–42362. - PubMed
-
- An, S., Musier-Forsyth, K. Cys-tRNA(Pro) editing by Haemophilus influenzae YbaK via a novel synthetase.YbaK.tRNA ternary complex. J. Biol. Chem. 2005;280:34465–34472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

