The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development
- PMID: 18765830
- PMCID: PMC3538864
- DOI: 10.1126/scisignal.1159945
The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development
Abstract
The extracellular Ca(2+)-sensing receptor (CaSR) plays a nonredundant role in the functions of the parathyroid gland (PTG) and the kidney. Severe hyperparathyroidism, premature death, and incomplete gene excision in Casr(-/-) mice have precluded the assessment of CaSR function in other tissues. We generated mice with tissue-specific deletion of Casr in the PTG, bone, or cartilage. Deletion of Casr in the PTG or bone resulted in profound bone defects, whereas deletion of Casr in chondrocytes (cartilage-producing cells) resulted in death before embryonic day 13 (E13). Mice in which chondrocyte-specific deletion of Casr was induced between E16 and E18 were viable but showed delayed growth plate development. Our data show a critical role for the CaSR in early embryogenesis and skeletal development.
Figures

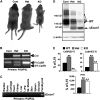


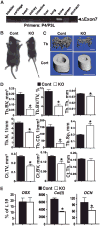


Comment in
-
New insights in bone biology: unmasking skeletal effects of the extracellular calcium-sensing receptor.Sci Signal. 2008 Sep 2;1(35):pe40. doi: 10.1126/scisignal.135pe40. Sci Signal. 2008. PMID: 18765829
References
-
- Brown EM. The calcium-sensing receptor: Physiology, pathophysiology and CaR-based therapeutics. Subcell Biochem. 2007;45:139–167. - PubMed
-
- Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. - PubMed
-
- Chang W, Shoback D. Extracellular Ca2+-sensing receptors—an overview. Cell Calcium. 2004;35:183–196. - PubMed
-
- Brown EM, Bai M, Pollak M. Familial benign hypocalciuric hypercalcemia and other syndromes of altered responsiveness to extracellular calcium. In: Avioli LV, Krane SM, editors. Metabolic Bone Disease. Academic Press; New York: 1998. pp. 479–499.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

