Choroideremia: analysis of the retina from a female symptomatic carrier
- PMID: 18766988
- PMCID: PMC3652314
- DOI: 10.1080/13816810802206499
Choroideremia: analysis of the retina from a female symptomatic carrier
Abstract
Purpose: To define the retinal pathology in a 91 year-old affected matriarch of a three-generation choroideremia family with multiple manifesting carriers.
Methods: Tissue from three different retinal areas was processed for immunohistochemistry. The macular area was processed for transmission electron microscopy. Cryosections were studied by indirect immunofluorescence, using well-characterized antibodies to cone cytoplasm, rhodopsin and cone opsins. The affected donor eyes were compared to a postmortem matched normal eye.
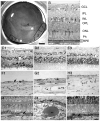
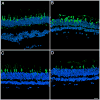
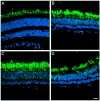
Results: The retina displayed areas of severe degeneration, with no photoreceptor outer segments, photoreceptor nuclear atrophy, and atrophy of the inner retina. Other retinal areas were near to normal. The RPE was severely degenerated, with thinning, pigment clumping and sub-epithelial debris deposition in all the areas examined. The choroid displayed depigmentation. Labeling with cone opsin antibodies revealed that cones were drastically affected: blue opsin was almost completely absent, while red/green opsins were distributed along the entire plasma membrane of the cell. Rhodopsin was also distributed along the entire rod plasma membrane. Ultrastructural analysis of the affected macula revealed the absence of RPE apical microvilli and basal infoldings. Instead, RPE's basal surface and choroid displayed the presence of banded fibers composed of clumps of wide-spacing collagen. Bruch's membrane was filled with vesicular structures, some smooth and others with bristle-like projections.
Conclusions: The histological data suggests that the clinical manifestation in this donor is related to degenerative changes in the retina, RPE, and choroid.
Figures




References
-
- Lyon M. Gene action in X chromosome of mouse (Mus musculus L.) Nature. 1961;190:372–373. - PubMed
-
- Endo K, Yuzawa M, Ohba N. Choroideremia associated with subretinal neovascular membrane. Acta Ophthalmol Scand. 2000;78:483–6. - PubMed
-
- Potter MJ, Wong E, Szabo SM, McTaggart KE. Clinical findings in a carrier of a new mutation in the choroideremia gene. Ophthalmology. 2004;111:1905–1909. - PubMed
-
- Rubin ML, Fishman RS, McKay RA. Choroideremia: study of a family and literature review. Arch Ophthalmol. 1966;76:563–574. - PubMed
-
- Mura M, Sereda C, Jablonski MM, MacDonald IM, Iannaccone A. Clinical and functional findings in choroideremia due to complete deletion of the CHM gene. Arch Ophthalmol. 2007;125:1107–1113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
