Redundant mechanisms recruit actin into the contractile ring in silkworm spermatocytes
- PMID: 18767903
- PMCID: PMC2528054
- DOI: 10.1371/journal.pbio.0060209
Redundant mechanisms recruit actin into the contractile ring in silkworm spermatocytes
Abstract
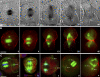
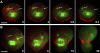
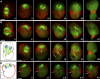
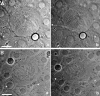
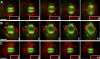
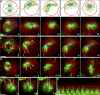
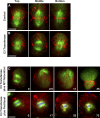
Cytokinesis is powered by the contraction of actomyosin filaments within the newly assembled contractile ring. Microtubules are a spindle component that is essential for the induction of cytokinesis. This induction could use central spindle and/or astral microtubules to stimulate cortical contraction around the spindle equator (equatorial stimulation). Alternatively, or in addition, induction could rely on astral microtubules to relax the polar cortex (polar relaxation). To investigate the relationship between microtubules, cortical stiffness, and contractile ring assembly, we used different configurations of microtubules to manipulate the distribution of actin in living silkworm spermatocytes. Mechanically repositioned, noninterdigitating microtubules can induce redistribution of actin at any region of the cortex by locally excluding cortical actin filaments. This cortical flow of actin promotes regional relaxation while increasing tension elsewhere (normally at the equatorial cortex). In contrast, repositioned interdigitating microtubule bundles use a novel mechanism to induce local stimulation of contractility anywhere within the cortex; at the antiparallel plus ends of central spindle microtubules, actin aggregates are rapidly assembled de novo and transported laterally to the equatorial cortex. Relaxation depends on microtubule dynamics but not on RhoA activity, whereas stimulation depends on RhoA activity but is largely independent of microtubule dynamics. We conclude that polar relaxation and equatorial stimulation mechanisms redundantly supply actin for contractile ring assembly, thus increasing the fidelity of cleavage.
Conflict of interest statement
Figures

Similar articles
-
Anillin interacts with microtubules and is part of the astral pathway that defines cortical domains.J Cell Sci. 2014 Sep 1;127(Pt 17):3699-710. doi: 10.1242/jcs.147504. Epub 2014 Jul 2. J Cell Sci. 2014. PMID: 24994938
-
Redistribution of actin during assembly and reassembly of the contractile ring in grasshopper spermatocytes.PLoS One. 2009;4(3):e4892. doi: 10.1371/journal.pone.0004892. Epub 2009 Mar 16. PLoS One. 2009. PMID: 19287500 Free PMC article.
-
Astral microtubules physically redistribute cortical actin filaments to the incipient contractile ring.Cytoskeleton (Hoboken). 2012 Nov;69(11):983-91. doi: 10.1002/cm.21073. Epub 2012 Oct 8. Cytoskeleton (Hoboken). 2012. PMID: 23027710
-
Relationships between the central spindle and the contractile ring during cytokinesis in animal cells.Microsc Res Tech. 2000 Apr 15;49(2):202-8. doi: 10.1002/(SICI)1097-0029(20000415)49:2<202::AID-JEMT13>3.0.CO;2-8. Microsc Res Tech. 2000. PMID: 10816260 Review.
-
The actin cytoskeleton in spindle assembly and positioning.Trends Cell Biol. 2009 Apr;19(4):174-9. doi: 10.1016/j.tcb.2009.01.006. Epub 2009 Mar 13. Trends Cell Biol. 2009. PMID: 19285869 Review.
Cited by
-
Molecular mechanisms of contractile-ring constriction and membrane trafficking in cytokinesis.Biophys Rev. 2018 Dec;10(6):1649-1666. doi: 10.1007/s12551-018-0479-3. Epub 2018 Nov 17. Biophys Rev. 2018. PMID: 30448943 Free PMC article. Review.
-
Molecular networks linked by Moesin drive remodeling of the cell cortex during mitosis.J Cell Biol. 2011 Oct 3;195(1):99-112. doi: 10.1083/jcb.201106048. J Cell Biol. 2011. PMID: 21969469 Free PMC article.
-
Centralspindlin and chromosomal passenger complex behavior during normal and Rappaport furrow specification in echinoderm embryos.Cytoskeleton (Hoboken). 2012 Oct;69(10):840-53. doi: 10.1002/cm.21061. Epub 2012 Aug 28. Cytoskeleton (Hoboken). 2012. PMID: 22887753 Free PMC article.
-
Action at a distance during cytokinesis.J Cell Biol. 2009 Dec 14;187(6):831-45. doi: 10.1083/jcb.200907090. J Cell Biol. 2009. PMID: 20008563 Free PMC article.
-
Dividing the spoils of growth and the cell cycle: The fission yeast as a model for the study of cytokinesis.Cytoskeleton (Hoboken). 2011 Feb;68(2):69-88. doi: 10.1002/cm.20500. Cytoskeleton (Hoboken). 2011. PMID: 21246752 Free PMC article. Review.
References
-
- D'Avino PP, Savoian MS, Glover DM. Cleavage furrow formation and ingression during animal cytokinesis: a microtubule legacy. J Cell Sci. 2005;118:1549–1558. - PubMed
-
- Burgess DR, Chang F. Site selection for the cleavage furrow at cytokinesis. Trends Cell Biol. 2005;15:156–162. - PubMed
-
- Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: from parts list to mechanisms. Annu Rev Biochem. 2006;75:543–566. - PubMed
-
- Piekny A, Werner M, Glotzer M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 2005;15:651–658. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

