Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells
- PMID: 18768478
- PMCID: PMC2573064
- DOI: 10.1074/jbc.M802529200
Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells
Abstract
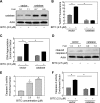
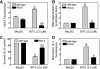
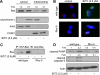
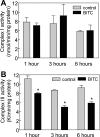
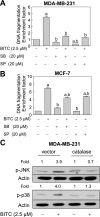
Benzyl isothiocyanate (BITC), a dietary cancer chemopreventive agent, causes apoptosis in MDA-MB-231 and MCF-7 human breast cancer cells, but the mechanism of cell death is not fully understood. We now demonstrate that the BITC-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species (ROS) due to inhibition of complex III of the mitochondrial respiratory chain. The BITC-induced ROS production and apoptosis were significantly inhibited by overexpression of catalase and Cu,Zn-superoxide dismutase and pharmacological inhibition of the mitochondrial respiratory chain. The mitochondrial DNA-deficient Rho-0 variant of MDA-MB-231 cells was nearly completely resistant to BITC-mediated ROS generation and apoptosis. The Rho-0 MDA-MB-231 cells also resisted BITC-mediated mitochondrial translocation (activation) of Bax. Biochemical assays revealed inhibition of complex III activity in BITC-treated MDA-MB-231 cells as early as at 1 h of treatment. The BITC treatment caused activation of c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK), which function upstream of Bax activation in apoptotic response to various stimuli. Pharmacological inhibition of both JNK and p38 MAPK conferred partial yet significant protection against BITC-induced apoptosis. Activation of JNK and p38 MAPK resulting from BITC exposure was abolished by overexpression of catalase. The BITC-mediated conformational change of Bax was markedly suppressed by ectopic expression of catalytically inactive mutant of JNK kinase 2 (JNKK2(AA)). Interestingly, a normal human mammary epithelial cell line was resistant to BITC-mediated ROS generation, JNK/p38 MAPK activation, and apoptosis. In conclusion, the present study indicates that the BITC-induced apoptosis in human breast cancer cells is initiated by mitochondria-derived ROS.
Figures






Similar articles
-
Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak.Mol Cancer Ther. 2006 Nov;5(11):2931-45. doi: 10.1158/1535-7163.MCT-06-0396. Mol Cancer Ther. 2006. PMID: 17121941
-
Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells.Carcinogenesis. 2009 Oct;30(10):1744-53. doi: 10.1093/carcin/bgp157. Epub 2009 Jun 23. Carcinogenesis. 2009. PMID: 19549704 Free PMC article.
-
Short-form RON overexpression augments benzyl isothiocyanate-induced apoptosis in human breast cancer cells.Mol Carcinog. 2016 May;55(5):473-85. doi: 10.1002/mc.22295. Epub 2015 Apr 7. Mol Carcinog. 2016. PMID: 25857724 Free PMC article.
-
Benzyl isothiocyanate (BITC) induces G2/M phase arrest and apoptosis in human melanoma A375.S2 cells through reactive oxygen species (ROS) and both mitochondria-dependent and death receptor-mediated multiple signaling pathways.J Agric Food Chem. 2012 Jan 18;60(2):665-75. doi: 10.1021/jf204193v. Epub 2012 Jan 6. J Agric Food Chem. 2012. PMID: 22148415
-
Sophorolipids as anticancer agents: progress and challenges.Discov Oncol. 2025 Apr 10;16(1):507. doi: 10.1007/s12672-025-02303-x. Discov Oncol. 2025. PMID: 40208440 Free PMC article. Review.
Cited by
-
Critical role of p53 upregulated modulator of apoptosis in benzyl isothiocyanate-induced apoptotic cell death.PLoS One. 2012;7(2):e32267. doi: 10.1371/journal.pone.0032267. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359675 Free PMC article.
-
Isothiocyanates induce oxidative stress and suppress the metastasis potential of human non-small cell lung cancer cells.BMC Cancer. 2010 Jun 9;10:269. doi: 10.1186/1471-2407-10-269. BMC Cancer. 2010. PMID: 20534110 Free PMC article.
-
Proteins as binding targets of isothiocyanates in cancer prevention.Carcinogenesis. 2011 Oct;32(10):1405-13. doi: 10.1093/carcin/bgr111. Epub 2011 Jun 10. Carcinogenesis. 2011. PMID: 21665889 Free PMC article. Review.
-
Mitochondrial dysfunction in cancer chemoprevention by phytochemicals from dietary and medicinal plants.Semin Cancer Biol. 2017 Dec;47:147-153. doi: 10.1016/j.semcancer.2016.11.009. Epub 2016 Nov 17. Semin Cancer Biol. 2017. PMID: 27867044 Free PMC article. Review.
-
Notch2 activation by benzyl isothiocyanate impedes its inhibitory effect on breast cancer cell migration.Breast Cancer Res Treat. 2012 Aug;134(3):1067-79. doi: 10.1007/s10549-012-2043-3. Epub 2012 Apr 4. Breast Cancer Res Treat. 2012. PMID: 22476855 Free PMC article.
References
-
- Jemal, A., Siegel, R., Ward, E., Murray, T., Xu, J., Smigal, C., and Thun, M. J. (2006) CA-Cancer J. Clin. 56 106-130 - PubMed
-
- Kelsey, J. L., Gammon, M. D., and John, E. M. (1993) Epidemiol. Rev. 15 36-47 - PubMed
-
- Hulka, B. S., and Stark, A. T. (1995) Lancet 346 883-887 - PubMed
-
- Hankinson, S. E., Willett, W. C., Manson, J. E., Colditz, G. A., Hunter, D. J., Spiegelman, D., Barbieri, R. L., and Speizer, F. E. (1998) J. Natl. Cancer Inst. 90 1292-1299 - PubMed
-
- Cauley, J. A., Lucas, F. L., Kuller, L. H., Stone, K., Browner, W., and Cummings, S. R. (1999) Ann. Intern. Med. 130 270-277 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

