Receptive field shift and shrinkage in macaque middle temporal area through attentional gain modulation
- PMID: 18768687
- PMCID: PMC6670861
- DOI: 10.1523/JNEUROSCI.4030-07.2008
Receptive field shift and shrinkage in macaque middle temporal area through attentional gain modulation
Abstract
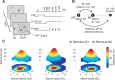
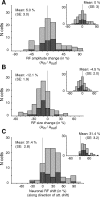
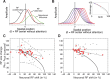
Selective attention is the top-down mechanism to allocate neuronal processing resources to the most relevant subset of the information provided by an organism's sensors. Attentional selection of a spatial location modulates the spatial-tuning characteristics (i.e., the receptive fields of neurons in macaque visual cortex). These tuning changes include a shift of receptive field centers toward the focus of attention and a narrowing of the receptive field when the attentional focus is directed into the receptive field. Here, we report that when attention is directed into versus of receptive fields of neurons in the middle temporal visual area (area MT), the magnitude of the shift of the spatial-tuning functions is positively correlated with a narrowing of spatial tuning around the attentional focus. By developing and applying a general attentional gain model, we show that these nonmultiplicative attentional modulations of basic neuronal-tuning characteristics could be a direct consequence of a spatially distributed multiplicative interaction of a bell-shaped attentional spotlight with the spatially fined-grained sensory inputs of MT neurons. Additionally, the model lets us estimate the spatial spread of the attentional top-down signal impinging on visual cortex. Consistent with psychophysical reports, the estimated size of the "spotlight of attention" indicates a coarse spatial resolution of attention. These results illustrate how spatially specific nonmultiplicative attentional changes of neuronal-tuning functions can be the result of multiplicative gain modulation affecting sensory neurons in a widely distributed region in cortical space.
Figures



References
-
- Andersen RA, Essick GK, Siegel RM. Encoding of spatial location by posterior parietal neurons. Science. 1985;230:456–458. - PubMed
-
- Bichot NP, Rossi AF, Desimone R. Parallel and serial neural mechanisms for visual search in macaque area V4. Science. 2005;308:529–534. - PubMed
-
- Boynton GM. Attention and visual perception. Curr Opin Neurobiol. 2005;15:465–469. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
