Sex-specific programming of offspring emotionality after stress early in pregnancy
- PMID: 18768700
- PMCID: PMC2731562
- DOI: 10.1523/JNEUROSCI.1424-08.2008
Sex-specific programming of offspring emotionality after stress early in pregnancy
Abstract
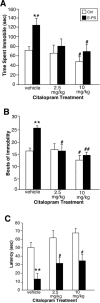
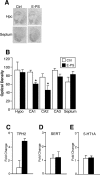
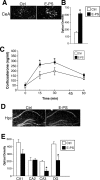
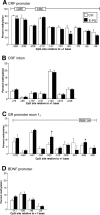
Prenatal stress is associated with an increased vulnerability to neurodevelopmental disorders, including autism and schizophrenia. To determine the critical time window when fetal antecedents may induce a disease predisposition, we examined behavioral responses in offspring exposed to stress during early, mid, and late gestation. We found that male offspring exposed to stress early in gestation displayed maladaptive behavioral stress responsivity, anhedonia, and an increased sensitivity to selective serotonin reuptake inhibitor treatment. Long-term alterations in central corticotropin-releasing factor (CRF) and glucocorticoid receptor (GR) expression, as well as increased hypothalamic-pituitary-adrenal (HPA) axis responsivity, were present in these mice and likely contributed to an elevated stress sensitivity. Changes in CRF and GR gene methylation correlated with altered gene expression, providing important evidence of epigenetic programming during early prenatal stress. In addition, we found the core mechanism underlying male vulnerability may involve sex-specific placenta responsivity, where stress early in pregnancy significantly increased expression of PPARalpha (peroxisome proliferator-activated receptor alpha), IGFBP-1 (insulin-like growth factor binding protein 1), HIF3alpha (hypoxia-inducible factor 3a), and GLUT4 (glucose transporter 4) in male placentas but not females. Examination of placental epigenetic machinery revealed basal sex differences, providing further evidence that sex-specific programming begins very early in pregnancy, and may contribute to the timing and vulnerability of the developing fetus to maternal perturbations. Overall, these results indicate that stress experience early in pregnancy may contribute to male neurodevelopmental disorders through impacts on placental function and fetal development.
Figures



References
-
- Altay T, Gonzales ER, Park TS, Gidday JM. Cerebrovascular inflammation after brief episodic hypoxia: modulation by neuronal and endothelial nitric oxide synthase. J Appl Physiol. 2004;96:1223–1230. discussion 1196. - PubMed
-
- Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006;139:813–820. - PubMed
-
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. - PubMed
-
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. - PubMed
-
- Bale TL, Anderson KR, Roberts AJ, Lee KF, Nagy TR, Vale WW. Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology. 2003;144:2580–2587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
