A microRNA DNA methylation signature for human cancer metastasis
- PMID: 18768788
- PMCID: PMC2528872
- DOI: 10.1073/pnas.0803055105
A microRNA DNA methylation signature for human cancer metastasis
Abstract
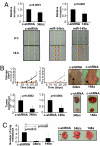
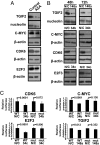
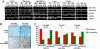
MicroRNAs (miRNAs) are small, noncoding RNAs that can contribute to cancer development and progression by acting as oncogenes or tumor suppressor genes. Recent studies have also linked different sets of miRNAs to metastasis through either the promotion or suppression of this malignant process. Interestingly, epigenetic silencing of miRNAs with tumor suppressor features by CpG island hypermethylation is also emerging as a common hallmark of human tumors. Thus, we wondered whether there was a miRNA hypermethylation profile characteristic of human metastasis. We used a pharmacological and genomic approach to reveal this aberrant epigenetic silencing program by treating lymph node metastatic cancer cells with a DNA demethylating agent followed by hybridization to an expression microarray. Among the miRNAs that were reactivated upon drug treatment, miR-148a, miR-34b/c, and miR-9 were found to undergo specific hypermethylation-associated silencing in cancer cells compared with normal tissues. The reintroduction of miR-148a and miR-34b/c in cancer cells with epigenetic inactivation inhibited their motility, reduced tumor growth, and inhibited metastasis formation in xenograft models, with an associated down-regulation of the miRNA oncogenic target genes, such as C-MYC, E2F3, CDK6, and TGIF2. Most important, the involvement of miR-148a, miR-34b/c, and miR-9 hypermethylation in metastasis formation was also suggested in human primary malignancies (n = 207) because it was significantly associated with the appearance of lymph node metastasis. Our findings indicate that DNA methylation-associated silencing of tumor suppressor miRNAs contributes to the development of human cancer metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Identification of microRNAs caused by DNA methylation that induce metastasis.Future Oncol. 2008 Dec;4(6):775-7. doi: 10.2217/14796694.4.6.775. Future Oncol. 2008. PMID: 19086843
References
-
- He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. - PubMed
-
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. - PubMed
-
- Esquela-Kerscher A, Slack FJ. OncomiRs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

