Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice
- PMID: 18768809
- PMCID: PMC2533226
- DOI: 10.1073/pnas.0800041105
Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice
Abstract
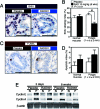
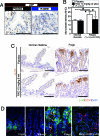
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that regulates cell growth via mTOR complex 1 (mTORC1), whose activation has been implicated in many human cancers. However, mTORC1's status in gastrointestinal tumors has not been characterized thoroughly. We have found that the mTORC1 pathway is activated with increased expression of the mTOR protein in intestinal polyps of the Apc(Delta716) heterozygous mutant mouse, a model for human familial adenomatous polyposis. An 8-week treatment with RAD001 (everolimus) suppressed the mTORC1 activity in these polyps and inhibited proliferation of the adenoma cells as well as tumor angiogenesis, which significantly reduced not only the number of polyps but also their size. beta-Catenin knockdown in the colon cancer cell lines reduced the mTOR level and thereby inhibited the mTORC1 signaling. These results suggest that the Wnt signaling contributes to mTORC1 activation through the increased level of mTOR and that the activation plays important roles in the intestinal polyp formation and growth. Indeed, long-term RAD001 treatment significantly reduced mortality of the Apc(Delta716) mice. Thus, we propose that the mTOR inhibitors may be efficacious for therapy and prevention of colonic adenomas and cancers with Wnt signaling activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. - PubMed
-
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. - PubMed
-
- Jacinto E, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. - PubMed
-
- Bjornsti M-A, Houghton PJ. The TOR pathway: A target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. - PubMed
-
- Majumder PK, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

