A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus
- PMID: 18768874
- PMCID: PMC2590646
- DOI: 10.4049/jimmunol.181.6.4168
A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus
Abstract
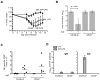
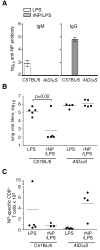
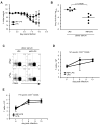
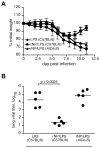
Current influenza vaccines elicit Abs to the hemagglutinin and neuraminidase envelope proteins. Due to antigenic drift, these vaccines must be reformulated annually to include the envelope proteins predicted to dominate in the following season. By contrast, vaccination with the conserved nucleoprotein (NP) elicits immunity against multiple serotypes (heterosubtypic immunity). NP vaccination is generally thought to convey protection primarily via CD8 effector mechanisms. However, significant titers of anti-NP Abs are also induced, yet the involvement of Abs in protection has largely been disregarded. To investigate how Ab responses might contribute to heterosubtypic immunity, we vaccinated C57BL/6 mice with soluble rNP. This approach induced high titers of NP-specific serum Ab, but only poorly detectable NP-specific T cell responses. Nevertheless, rNP immunization significantly reduced morbidity and viral titers after influenza challenge. Importantly, Ab-deficient mice were not protected by this vaccination strategy. Furthermore, rNP-immune serum could transfer protection to naive hosts in an Ab-dependent manner. Therefore, Ab to conserved, internal viral proteins, such as NP, provides an unexpected, yet important mechanism of protection against influenza. These results suggest that vaccines designed to elicit optimal heterosubtypic immunity to influenza should promote both Ab and T cell responses to conserved internal proteins.
Figures


References
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. Jama. 2004;292:1333–1340. - PubMed
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289:179–186. - PubMed
-
- Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. - PubMed
-
- Kaiser J. A one-size-fits-all flu vaccine? Science. 2006;312:380–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

