Rapid deletional peripheral CD8 T cell tolerance induced by allogeneic bone marrow: role of donor class II MHC and B cells
- PMID: 18768896
- PMCID: PMC2628539
- DOI: 10.4049/jimmunol.181.6.4371
Rapid deletional peripheral CD8 T cell tolerance induced by allogeneic bone marrow: role of donor class II MHC and B cells
Abstract
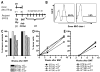
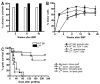
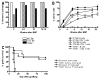
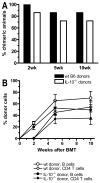
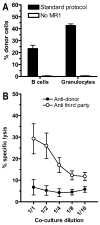
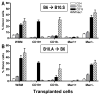
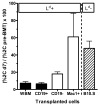
Mixed chimerism and donor-specific tolerance are achieved in mice receiving 3 Gy of total body irradiation and anti-CD154 mAb followed by allogeneic bone marrow (BM) transplantation. In this model, recipient CD4 cells are critically important for CD8 tolerance. To evaluate the role of CD4 cells recognizing donor MHC class II directly, we used class II-deficient donor marrow and were not able to achieve chimerism unless recipient CD8 cells were depleted, indicating that directly alloreactive CD4 cells were necessary for CD8 tolerance. To identify the MHC class II(+) donor cells promoting this tolerance, we used donor BM lacking certain cell populations or used positively selected cell populations. Neither donor CD11c(+) dendritic cells, B cells, T cells, nor donor-derived IL-10 were critical for chimerism induction. Purified donor B cells induced early chimerism and donor-specific cell-mediated lympholysis tolerance in both strain combinations tested. In contrast, positively selected CD11b(+) monocytes/myeloid cells did not induce early chimerism in either strain combination. Donor cell preparations containing B cells were able to induce early deletion of donor-reactive TCR-transgenic 2C CD8 T cells, whereas those devoid of B cells had reduced activity. Thus, induction of stable mixed chimerism depends on the expression of MHC class II on the donor marrow, but no requisite donor cell lineage was identified. Donor BM-derived B cells induced early chimerism, donor-specific cell-mediated lympholysis tolerance, and deletion of donor-reactive CD8 T cells, whereas CD11b(+) cells did not. Thus, BM-derived B cells are potent tolerogenic APCs for alloreactive CD8 cells.
Figures


References
-
- Sayegh MH, Fine NA, Smith JL, Rennke HG, Milford EL, Tilney NL. Immunologic tolerance to renal allografts after bone marrow transplants from the same donors. Ann Intern Med. 1991;114:954–955. - PubMed
-
- Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transpl Immunol. 2004;13:117–130. - PubMed
-
- Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, Sykes M, Monroy R, Tanaka M, Sachs DH. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

