Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease
- PMID: 18768899
- PMCID: PMC2775538
- DOI: 10.4049/jimmunol.181.6.4397
Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease
Abstract
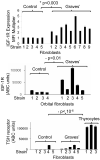
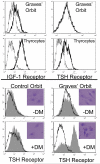
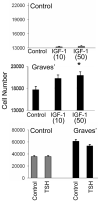
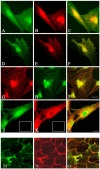
Thyroid-stimulating hormone receptor (TSHR) plays a central role in regulating thyroid function and is targeted by IgGs in Graves' disease (GD-IgG). Whether TSHR is involved in the pathogenesis of thyroid-associated ophthalmopathy (TAO), the orbital manifestation of GD, remains uncertain. TSHR signaling overlaps with that of insulin-like grow factor 1 receptor (IGF-1R). GD-IgG can activate fibroblasts derived from donors with GD to synthesize T cell chemoattractants and hyaluronan, actions mediated through IGF-1R. In this study, we compare levels of IGF-1R and TSHR on the surfaces of TAO and control orbital fibroblasts and thyrocytes and explore the physical and functional relationship between the two receptors. TSHR levels are 11-fold higher on thyrocytes than on TAO or control fibroblasts. In contrast, IGF-1R levels are 3-fold higher on TAO vs control fibroblasts. In pull-down studies using fibroblasts, thyrocytes, and thyroid tissue, Abs directed specifically against either IGF-1Rbeta or TSHR bring both proteins out of solution. Moreover, IGF-1Rbeta and TSHR colocalize to the perinuclear and cytoplasmic compartments in fibroblasts and thyrocytes by confocal microscopy. Examination of orbital tissue from patients with TAO reveals similar colocalization to cell membranes. Treatment of primary thyrocytes with recombinant human TSH results in rapid ERK phosphorylation which can be blocked by an IGF-1R-blocking mAb. Our findings suggest that IGF-1R might mediate some TSH-provoked signaling. Furthermore, they indicate that TSHR levels on orbital fibroblasts are considerably lower than those on thyrocytes and that this receptor associates with IGF-1R in situ and together may comprise a functional complex in thyroid and orbital tissue.
Figures


References
-
- De Meyts P, Whittaker J. Structural biology of insulin and IGF1 receptors: implications for drug design. Nature Rev. 2002;1:769–783. - PubMed
-
- Dupont J, Fernandez AM, Glackin CA, Helman L, LeRoith D. Insulin-like growth factor 1 (IGF-1)-induced twist expression is involved in the anti-apoptotic effects of the IGF-1 receptor. J. Biol. Chem. 2001;276:26699–26707. - PubMed
-
- Kooijman R, Scholtens LE, Rijkers GT, Zegers BJM. Differential expression of type I insulin-like growth factor receptors in different stages of human T cells. Eur. J. Immunol. 1995;25:931–935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

