The regulation of cell wall extensibility during shade avoidance: a study using two contrasting ecotypes of Stellaria longipes
- PMID: 18768908
- PMCID: PMC2577261
- DOI: 10.1104/pp.108.125518
The regulation of cell wall extensibility during shade avoidance: a study using two contrasting ecotypes of Stellaria longipes
Abstract
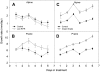
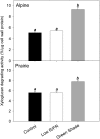
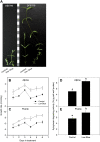
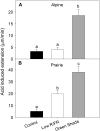
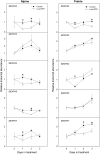
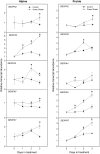
Shade avoidance in plants involves rapid shoot elongation to grow toward the light. Cell wall-modifying mechanisms are vital regulatory points for control of these elongation responses. Two protein families involved in cell wall modification are expansins and xyloglucan endotransglucosylase/hydrolases. We used an alpine and a prairie ecotype of Stellaria longipes differing in their response to shade to study the regulation of cell wall extensibility in response to low red to far-red ratio (R/FR), an early neighbor detection signal, and dense canopy shade (green shade: low R/FR, blue, and total light intensity). Alpine plants were nonresponsive to low R/FR, while prairie plants elongated rapidly. These responses reflect adaptation to the dense vegetation of the prairie habitat, unlike the alpine plants, which almost never encounter shade. Under green shade, both ecotypes rapidly elongate, showing that alpine plants can react only to a deep shade treatment. Xyloglucan endotransglucosylase/hydrolase activity was strongly regulated by green shade and low blue light conditions but not by low R/FR. Expansin activity, expressed as acid-induced extension, correlated with growth responses to all light changes. Expansin genes cloned from the internodes of the two ecotypes showed differential regulation in response to the light manipulations. This regulation was ecotype and light signal specific and correlated with the growth responses. Our results imply that elongation responses to shade require the regulation of cell wall extensibility via the control of expansin gene expression. Ecotypic differences demonstrate how responses to environmental stimuli are differently regulated to survive a particular habitat.
Figures

Comment in
-
A molecular basis for the physiological variation in shade avoidance responses: a tale of two ecotypes.Plant Signal Behav. 2009 Jun;4(6):528-9. doi: 10.1104/pp.108.125518. Epub 2009 Jun 30. Plant Signal Behav. 2009. PMID: 19816143 Free PMC article.
Similar articles
-
Light quality-mediated petiole elongation in Arabidopsis during shade avoidance involves cell wall modification by xyloglucan endotransglucosylase/hydrolases.Plant Physiol. 2010 Oct;154(2):978-90. doi: 10.1104/pp.110.162057. Epub 2010 Aug 5. Plant Physiol. 2010. PMID: 20688978 Free PMC article.
-
Phenotypic plasticity of sun and shade ecotypes of Stellaria longipes in response to light quality signaling, gibberellins and auxin.Plant Physiol Biochem. 2015 Sep;94:174-80. doi: 10.1016/j.plaphy.2015.06.013. Epub 2015 Jun 17. Plant Physiol Biochem. 2015. PMID: 26113156
-
Growth and ethylene evolution by shade and sun ecotypes of Stellaria longipes in response to varied light quality and irradiance.Plant Cell Environ. 2006 Apr;29(4):647-52. doi: 10.1111/j.1365-3040.2005.01443.x. Plant Cell Environ. 2006. PMID: 17080614
-
Blue light regulated shade avoidance.Plant Signal Behav. 2012 Apr;7(4):514-7. doi: 10.4161/psb.19340. Epub 2012 Apr 1. Plant Signal Behav. 2012. PMID: 22499181 Free PMC article. Review.
-
Plant adaptation to dynamically changing environment: the shade avoidance response.Biotechnol Adv. 2012 Sep-Oct;30(5):1047-58. doi: 10.1016/j.biotechadv.2011.08.014. Epub 2011 Aug 24. Biotechnol Adv. 2012. PMID: 21888962 Review.
Cited by
-
RhEXPA4, a rose expansin gene, modulates leaf growth and confers drought and salt tolerance to Arabidopsis.Planta. 2013 Jun;237(6):1547-59. doi: 10.1007/s00425-013-1867-3. Epub 2013 Mar 16. Planta. 2013. PMID: 23503758
-
Light quality-mediated petiole elongation in Arabidopsis during shade avoidance involves cell wall modification by xyloglucan endotransglucosylase/hydrolases.Plant Physiol. 2010 Oct;154(2):978-90. doi: 10.1104/pp.110.162057. Epub 2010 Aug 5. Plant Physiol. 2010. PMID: 20688978 Free PMC article.
-
Effect of Various LED Light Qualities, Including Wide Red Spectrum-LED, on the Growth and Quality of Mini Red Romaine Lettuce (cv. Breen).Plants (Basel). 2023 May 22;12(10):2056. doi: 10.3390/plants12102056. Plants (Basel). 2023. PMID: 37653973 Free PMC article.
-
Time-Course Transcriptomics Analysis Reveals Key Responses of Submerged Deepwater Rice to Flooding.Plant Physiol. 2018 Apr;176(4):3081-3102. doi: 10.1104/pp.17.00858. Epub 2018 Feb 23. Plant Physiol. 2018. PMID: 29475897 Free PMC article.
-
Karrikins delay soybean seed germination by mediating abscisic acid and gibberellin biogenesis under shaded conditions.Sci Rep. 2016 Feb 23;6:22073. doi: 10.1038/srep22073. Sci Rep. 2016. PMID: 26902640 Free PMC article.
References
-
- Albert M, Werner M, Proksch P, Fry SC, Kaldenhoff R (2004) The cell wall-modifying xyloglucan endotransglycosylase/hydrolase LeXTH1 is expressed during the defence reaction of tomato against the plant parasite Cuscuta reflexa. Plant Biol 6 402–407 - PubMed
-
- Alokam S, Chinnappa CC, Reid DM (2002) Red/far-red light mediated stem elongation and anthocyanin accumulation in Stellaria longipes: differential response of alpine and prairie ecotypes. Can J Bot 80 72–81
-
- Ballaré CL, Casal JJ, Kendrick RE (1991) Responses of light-grown wild-type and long-hypocotyl mutant cucumber seedlings to natural and stimulated shade light. Photochem Photobiol 54 819–826
-
- Ballaré CL, Scopel AL, Sanchéz RA (1990) Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247 329–332 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

