Protein tyrosine kinases and protein tyrosine phosphatases are involved in abscisic acid-dependent processes in Arabidopsis seeds and suspension cells
- PMID: 18768909
- PMCID: PMC2577241
- DOI: 10.1104/pp.108.124594
Protein tyrosine kinases and protein tyrosine phosphatases are involved in abscisic acid-dependent processes in Arabidopsis seeds and suspension cells
Abstract
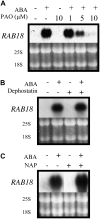
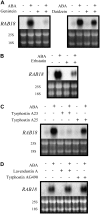
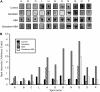
Protein tyrosine (Tyr) phosphorylation plays a central role in many signaling pathways leading to cell growth and differentiation in animals. Tyr phosphorylated proteins have been detected in higher plants, and the roles of protein Tyr phosphatases and protein Tyr kinases in some physiological responses have been shown. We investigated the involvement of Tyr phosphorylation events in abscisic acid (ABA) signaling using a pharmacological approach. Phenylarsine oxide, a specific inhibitor of protein Tyr phosphatase activity, abolished the ABA-dependent accumulation of RAB18 (responsive to ABA 18) transcripts. Protein Tyr kinase inhibitors like genistein, tyrphostin A23, and erbstatin blocked the RAB18 expression induced by ABA in Arabidopsis (Arabidopsis thaliana). Stomatal closure induced by ABA was also inhibited by phenylarsine oxide and genistein. We studied the changes in the Tyr phosphorylation levels of proteins in Arabidopsis seeds after ABA treatment. Proteins were separated by two-dimensional gel electrophoresis, and those phosphorylated on Tyr residues were detected using an anti-phosphotyrosine antibody by western blot. Changes were detected in the Tyr phosphorylation levels of 19 proteins after ABA treatment. Genistein inhibited the ABA-dependent Tyr phosphorylation of proteins. The 19 proteins were analyzed by matrix-assisted laser-desorption ionization time-of-flight/time-of-flight mass spectrometry. Among the proteins identified were storage proteins like cruciferins, enzymes involved in the mobilization of lipid reserves like aconitase, enolase, aldolase, and a lipoprotein, and enzymes necessary for seedling development like the large subunit of Rubisco. Additionally, the identification of three putative signaling proteins, a peptidyl-prolyl isomerase, an RNA-binding protein, and a small ubiquitin-like modifier-conjugating enzyme, enlightens how Tyr phosphorylation might regulate ABA transduction pathways in plants.
Figures



Similar articles
-
Evidence of a role for tyrosine dephosphorylation in the control of postgermination arrest of development by abscisic acid in Arabidopsis thaliana L.Planta. 2006 Jan;223(2):381-5. doi: 10.1007/s00425-005-0135-6. Epub 2005 Oct 7. Planta. 2006. PMID: 16211388
-
Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins.J Physiol. 1998 Jan 15;506 ( Pt 2)(Pt 2):341-52. doi: 10.1111/j.1469-7793.1998.341bw.x. J Physiol. 1998. PMID: 9490863 Free PMC article.
-
Effects of tyrosine kinase and phosphatase inhibitors on microtubules in Arabidopsis root cells.Cell Biol Int. 2008 Jun;32(6):630-7. doi: 10.1016/j.cellbi.2008.01.013. Epub 2008 Jan 25. Cell Biol Int. 2008. PMID: 18343165
-
Protein phosphorylation in stomatal movement.Plant Signal Behav. 2014;9(11):e972845. doi: 10.4161/15592316.2014.972845. Plant Signal Behav. 2014. PMID: 25482764 Free PMC article. Review.
-
Signal processing by protein tyrosine phosphorylation in plants.Plant Signal Behav. 2011 Jul;6(7):942-51. doi: 10.4161/psb.6.7.15261. Plant Signal Behav. 2011. PMID: 21628997 Free PMC article. Review.
Cited by
-
Advances in the Biology of Seed and Vegetative Storage Proteins Based on Two-Dimensional Electrophoresis Coupled to Mass Spectrometry.Molecules. 2018 Sep 26;23(10):2462. doi: 10.3390/molecules23102462. Molecules. 2018. PMID: 30261600 Free PMC article. Review.
-
Inhibition of Arabidopsis O-acetylserine(thiol)lyase A1 by tyrosine nitration.J Biol Chem. 2011 Jan 7;286(1):578-86. doi: 10.1074/jbc.M110.147678. Epub 2010 Nov 3. J Biol Chem. 2011. PMID: 21047785 Free PMC article.
-
Sexual and Apogamous Species of Woodferns Show Different Protein and Phytohormone Profiles.Front Plant Sci. 2021 Nov 12;12:718932. doi: 10.3389/fpls.2021.718932. eCollection 2021. Front Plant Sci. 2021. PMID: 34868105 Free PMC article.
-
ABA signalling manipulation suppresses senescence of a leafy vegetable stored at room temperature.Plant Biotechnol J. 2018 Feb;16(2):530-544. doi: 10.1111/pbi.12793. Epub 2017 Aug 16. Plant Biotechnol J. 2018. PMID: 28703416 Free PMC article.
-
Methyl Syringate Stimulates Glucose Uptake by Inhibiting Protein Tyrosine Phosphatases Relevant to Insulin Resistance.Life (Basel). 2023 Jun 12;13(6):1372. doi: 10.3390/life13061372. Life (Basel). 2023. PMID: 37374154 Free PMC article.
References
-
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe SI, Itoh N, Shibuya M, Fukami Y (1987) Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262 5592–5595 - PubMed
-
- Ali N, Halfter U, Chua NH (1994) Cloning and biochemical characterization of a plant protein kinase that phosphorylates serine, threonine and tyrosine. J Biol Chem 269 31626–31629 - PubMed
-
- Axelos M, Curie C, Mazzolini L, Bardet C, Lescure B (1992) A protocol for transient gene expression in Arabidopsis thaliana protoplasts isolated from cell suspension cultures. Plant Physiol Biochem 30 123–128
-
- Barizza E, Sciavo FL, Terzi M, Filippini F (1999) Evidence suggesting protein tyrosine phosphorylation in plants depends on the developmental conditions. FEBS Lett 447 191–194 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

