Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line
- PMID: 18768927
- PMCID: PMC2584989
- DOI: 10.1152/ajpcell.00144.2008
Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line
Abstract
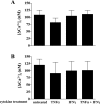
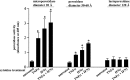
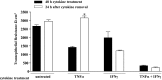
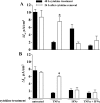
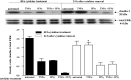
Sjögren's syndrome (SS) is an autoimmune disorder characterized by inflammation and dysfunction of salivary glands, resulting in impaired secretory function. The production of the proinflammatory cytokines tumor necrosis factor-alpha (TNF-alpha) and interferon-gamma (IFN-gamma) is elevated in exocrine glands of patients with SS, although little is known about the effects of these cytokines on salivary epithelial cell functions necessary for saliva secretion, including tight junction (TJ) integrity and the establishment of transepithelial ion gradients. The present study demonstrates that chronic exposure of polarized rat parotid gland (Par-C10) epithelial cell monolayers to TNF-alpha and IFN-gamma decreases transepithelial resistance (TER) and anion secretion, as measured by changes in short-circuit current (I(sc)) induced by carbachol, a muscarinic cholinergic receptor agonist, or UTP, a P2Y(2) nucleotide receptor agonist. In contrast, TNF-alpha and IFN-gamma had no effect on agonist-induced increases in the intracellular calcium concentration [Ca(2+)](i) in Par-C10 cells. Furthermore, treatment of Par-C10 cell monolayers with TNF-alpha and IFN-gamma increased paracellular permeability to normally impermeant proteins, altered cell and TJ morphology, and downregulated the expression of the TJ protein, claudin-1, but not other TJ proteins expressed in Par-C10 cells. The decreases in TER, agonist-induced transepithelial anion secretion, and claudin-1 expression caused by TNF-alpha, but not IFN-gamma, were reversible by incubation of Par-C10 cell monolayers with cytokine-free medium for 24 h, indicating that IFN-gamma causes irreversible inhibition of cellular activities associated with fluid secretion in salivary glands. Our results suggest that cytokine production is an important contributor to secretory dysfunction in SS by disrupting TJ integrity of salivary epithelium.
Figures





References
-
- Adams RB, Planchon SM, Roche JK. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol 150: 2356–2363, 1993. - PubMed
-
- Adamson TC, Fox RI, Frisman DM, Howell FV. Immunohistologic analysis of lymphoid infiltrates in primary Sjögren's syndrome using monoclonal antibodies. J Immunol 130: 203–208, 1983. - PubMed
-
- Akao S, Kiumi F. The tight junction of main pancreatic duct epithelial cells is a morphometrically dynamic structure altered by intraductal hypertension. Med Electron Microsc 35: 146–152, 2002. - PubMed
-
- Awasthi N, Wagner BJ. Interferon-gamma induces apoptosis of lens alphaTN4–1 cells and proteasome inhibition has an antiapoptotic effect. Invest Ophthalmol Vis Sci 45: 222–229, 2004. - PubMed
-
- Baggaley E, McLarnon S, Demeter I, Varga G, Bruce JI. Differential regulation of the apical plasma membrane Ca2+-ATPase by protein kinase A in parotid acinar cells. J Biol Chem 282: 37678–37693, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

