Within-host genetic diversity of endemic and emerging parvoviruses of dogs and cats
- PMID: 18768982
- PMCID: PMC2573264
- DOI: 10.1128/JVI.01003-08
Within-host genetic diversity of endemic and emerging parvoviruses of dogs and cats
Abstract
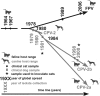
Viral emergence can result from the adaptation of endemic pathogens to new or altered host environments, a process that is strongly influenced by the underlying sequence diversity. To determine the extent and structure of intrahost genetic diversity in a recently emerged single-stranded DNA virus, we analyzed viral population structures during natural infections of animals with canine parvovirus (CPV) or its ancestor, feline panleukopenia virus (FPV). We compared infections that occurred shortly after CPV emerged with more recent infections and examined the population structure of CPV after experimental cross-species transmission to cats. Infections with CPV and FPV showed limited genetic diversity regardless of the analyzed host tissue or year of isolation. Coinfections with genetically distinct viral strains were detected in some cases, and rearranged genomes were seen in both FPV and CPV. The sporadic presence of some sequences with multiple mutations suggested the occurrence of either particularly error-prone viral replication or coinfection by more distantly related strains. Finally, some potentially organ-specific host effects were seen during experimental cross-species transmission, with many of the mutations located in the nonstructural protein NS2. These included residues with evidence of positive selection at the population level, which is compatible with a role of this protein in host adaptation.
Figures


References
-
- Battilani, M., L. Gallina, F. Vaccari, and L. Morganti. 2007. Co-infection with multiple variants of canine parvovirus type 2 (CPV-2). Vet. Res. Commun. 31(Suppl. 1)209-212. - PubMed
-
- Battilani, M., A. Scagliarini, S. Ciulli, L. Morganti, and S. Prosperi. 2006. High genetic diversity of the VP2 gene of a canine parvovirus strain detected in a domestic cat. Virology 35222-26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

