The yin and yang of the Cdkn2a locus in senescence and aging
- PMID: 18769141
- PMCID: PMC2987737
- DOI: 10.4161/cc.7.18.6687
The yin and yang of the Cdkn2a locus in senescence and aging
Abstract
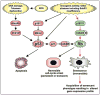
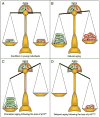
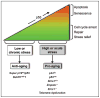
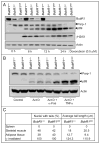
Senescence of cultured cells involves activation of the p19(Arf)-p53 and the p16(Ink4a)-Rb tumor suppressor pathways. This, together with the observation that p19(Arf) and p16(Ink4a) expression increases with age in many tissues of humans and rodents, led to the speculation that these pathways drive in vivo senescence and natural aging. However, it has been difficult to test this hypothesis using a mammalian model system because inactivation of either of these pathways results in early death from tumors. One approach to bypass this problem would be to inactivate these pathways in a murine segmental progeria model such as mice that express low amounts of the mitotic checkpoint protein BubR1 (BubR1 hypomorphic mice). These mice have a five-fold reduced lifespan and develop a variety of early-aging associated phenotypes including cachetic dwarfism, skeletal muscle degeneration, cataracts, arterial stiffening, (subcutaneous) fat loss, reduced stress tolerance and impaired wound healing. Importantly, BubR1 hypomorphism elevates both p16(Ink4a) and p19(Arf) expression in skeletal muscle and fat. Inactivation of p16(Ink4a) in BubR1 mutant mice delays both cellular senescence and aging specifically in these tissues. Surprisingly, however, inactivation of p19(Arf) has the opposite effect; it exacerbates in vivo senescence and aging in skeletal muscle and fat. These mouse studies suggest that p16(Ink4a) is indeed an effector of aging and in vivo senescence, but p19(Arf) an attenuator. Thus, the role of the p19(Arf)-p53 pathway in aging and in vivo senescence seems far more complex than previously anticipated.
Figures

References
-
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33. - PubMed
-
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40. - PubMed
-
- Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, Grosveld G, Sherr CJ. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–59. - PubMed
-
- Sherr CJ. The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol. 2001;2:731–7. - PubMed
-
- Campisi J. Senescent cells, tumor suppression and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
