Development and Notch signaling requirements of the zebrafish choroid plexus
- PMID: 18769591
- PMCID: PMC2528000
- DOI: 10.1371/journal.pone.0003114
Development and Notch signaling requirements of the zebrafish choroid plexus
Abstract
Background: The choroid plexus (CP) is an epithelial and vascular structure in the ventricular system of the brain that is a critical part of the blood-brain barrier. The CP has two primary functions, 1) to produce and regulate components of the cerebral spinal fluid, and 2) to inhibit entry into the brain of exogenous substances. Despite its importance in neurobiology, little is known about how this structure forms.
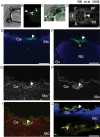
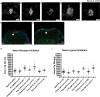
Methodology and principal findings: Here we show that the transposon-mediated enhancer trap zebrafish line Et(Mn16) expresses green fluorescent protein within a population of cells that migrate toward the midline and coalesce to form the definitive CP. We further demonstrate the development of the integral vascular network of the definitive CP. Utilizing pharmacologic pan-notch inhibition and specific morpholino-mediated knockdown, we demonstrate a requirement for Notch signaling in choroid plexus development. We identify three Notch signaling pathway members as mediating this effect, notch1b, deltaA, and deltaD.
Conclusions and significance: This work is the first to identify the zebrafish choroid plexus and to characterize its epithelial and vasculature integration. This study, in the context of other comparative anatomical studies, strongly indicates a conserved mechanism for development of the CP. Finally, we characterize a requirement for Notch signaling in the developing CP. This establishes the zebrafish CP as an important new system for the determination of key signaling pathways in the formation of this essential component of the vertebrate brain.
Conflict of interest statement
Figures



References
-
- Netsky MG, Shuangshoti S, Tennyson VM, Brightman MW, Becker NH, et al. The Choroid Plexus in Health and Disease. Charlottesville, VA, USA: University Press of Virginia; 1975.
-
- Strazielle N, Ghersi-Egea JF. Choroid plexus in the central nervous system: biology and physiopathology. J Neuropathol Exp Neurol. 2000;59:561–574. - PubMed
-
- Tennyson VM, Appas GD. The fine structure of the choroid plexus adult and developmental stages. Prog Brain Res. 1968;29:63–85. - PubMed
-
- Jacobsen M, Clausen PP, Jacobsen GK, Saunders NR, Mollgard K. Intracellular plasma proteins in human fetal choroid plexus during development. I. Developmental stages in relation to the number of epithelial cells which contain albumin in telencephalic, diencephalic and myelencephalic choroid plexus. Brain Res. 1982;255:239–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

