B cell antigen presentation promotes Th2 responses and immunopathology during chronic allergic lung disease
- PMID: 18769622
- PMCID: PMC2518863
- DOI: 10.1371/journal.pone.0003129
B cell antigen presentation promotes Th2 responses and immunopathology during chronic allergic lung disease
Abstract
Background: The role of B cells in allergic asthma remains undefined. One mechanism by which B cells clearly contribute to allergic disease is via the production of specific immunoglobulin, and especially IgE. Cognate interactions with specific T cells result in T cell help for B cells, resulting in differentiation and immunoglobulin secretion. Proximal to (and required for) T cell-dependent immunoglobulin production, however, is antigen presentation by B cells. While interaction with T cells clearly has implications for B cell function and differentiation, this study investigated the role that B cells have in shaping the T cell response during chronic allergic lung disease.
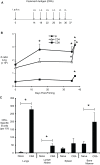
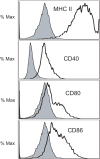
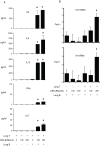
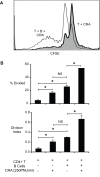
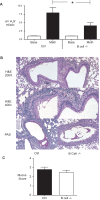
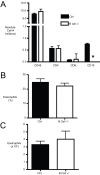
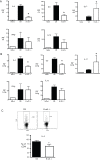
Methodology/principal findings: In these studies, we used a clinically relevant mouse model of chronic allergic lung disease to study the role of B cells and B cell antigen presentation in this disease. In these studies we present several novel findings: 1) Lung B cells from chronically allergen challenged mice up-regulated MHC II and costimulatory molecules CD40, CD80 and CD86. 2) Using in vitro studies, B cells from the lungs of allergen challenged mice could present antigen to T cells, as assessed by T cell proliferation and the preferential production of Th2 cytokines. 3) Following chronic allergen challenge, the levels of Th2 cytokines IL-4 and IL-5 in the lungs and airways were significantly attenuated in B cell -/- mice, relative to controls. 4) B cell driven Th2 responses and mucus hyper secretion in the lungs were dependent upon MHC II expression by B cells.
Conclusions/significance: Collectively, these results provide evidence for antigen presentation as a novel mechanism by which B cells contribute to chronic allergic disease. These findings give new insight into the mechanisms by which B cells promote asthma and other chronic diseases.
Conflict of interest statement
Figures

Similar articles
-
House dust mite-driven asthma and allergen-specific T cells depend on B cells when the amount of inhaled allergen is limiting.J Allergy Clin Immunol. 2017 Jul;140(1):76-88.e7. doi: 10.1016/j.jaci.2016.09.020. Epub 2016 Oct 13. J Allergy Clin Immunol. 2017. PMID: 27746238
-
Role of B cells in TH cell responses in a mouse model of asthma.J Allergy Clin Immunol. 2018 Apr;141(4):1395-1410. doi: 10.1016/j.jaci.2017.09.001. Epub 2017 Sep 7. J Allergy Clin Immunol. 2018. PMID: 28889953 Free PMC article.
-
Immunostimulatory oligonucleotides block allergic airway inflammation by inhibiting Th2 cell activation and IgE-mediated cytokine induction.J Exp Med. 2005 Dec 5;202(11):1563-73. doi: 10.1084/jem.20050631. Epub 2005 Nov 28. J Exp Med. 2005. PMID: 16314434 Free PMC article.
-
Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses.Front Immunol. 2018 Mar 19;9:455. doi: 10.3389/fimmu.2018.00455. eCollection 2018. Front Immunol. 2018. PMID: 29616018 Free PMC article. Review.
-
Modulation of the human IgE response.Eur Respir J Suppl. 1996 Aug;22:58s-62s. Eur Respir J Suppl. 1996. PMID: 8871045 Review.
Cited by
-
Initiation and Pathogenesis of Severe Asthma with Fungal Sensitization.Cells. 2021 Apr 15;10(4):913. doi: 10.3390/cells10040913. Cells. 2021. PMID: 33921169 Free PMC article. Review.
-
Exosomes in Severe Asthma: Update in Their Roles and Potential in Therapy.Biomed Res Int. 2018 May 8;2018:2862187. doi: 10.1155/2018/2862187. eCollection 2018. Biomed Res Int. 2018. PMID: 29854739 Free PMC article. Review.
-
T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite.Immunity. 2016 Feb 16;44(2):259-73. doi: 10.1016/j.immuni.2015.11.017. Epub 2016 Jan 26. Immunity. 2016. PMID: 26825674 Free PMC article.
-
CD36 and Platelet-Activating Factor Receptor Promote House Dust Mite Allergy Development.J Immunol. 2017 Aug 1;199(3):1184-1195. doi: 10.4049/jimmunol.1700034. Epub 2017 Jun 30. J Immunol. 2017. PMID: 28667161 Free PMC article.
-
B Cell Responses: Cell Interaction Dynamics and Decisions.Cell. 2019 Apr 18;177(3):524-540. doi: 10.1016/j.cell.2019.03.016. Cell. 2019. PMID: 31002794 Free PMC article. Review.
References
-
- Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, et al. Surveillance for asthma–United States, 1980–1999. MMWR Surveill Summ. 2002;51:1–13. - PubMed
-
- Aberg N, Hesselmar B, Aberg B, Eriksson B. Increase of asthma, allergic rhinitis and eczema in Swedish schoolchildren between 1979 and 1991. Clin Exp Allergy. 1995;25:815–819. - PubMed
-
- von Mutius E, Weiland SK, Fritzsch C, Duhme H, Keil U. Increasing prevalence of hay fever and atopy among children in Leipzig, East Germany. Lancet. 1998;351:862–866. - PubMed
-
- Garcia-Marcos L, Quiros AB, Hernandez GG, Guillen-Grima F, Diaz CG, et al. Stabilization of asthma prevalence among adolescents and increase among schoolchildren (ISAAC phases I and III) in Spain. Allergy. 2004;59:1301–1307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

