A C-terminal protease-resistant prion fragment distinguishes ovine "CH1641-like" scrapie from bovine classical and L-Type BSE in ovine transgenic mice
- PMID: 18769714
- PMCID: PMC2516186
- DOI: 10.1371/journal.ppat.1000137
A C-terminal protease-resistant prion fragment distinguishes ovine "CH1641-like" scrapie from bovine classical and L-Type BSE in ovine transgenic mice
Abstract
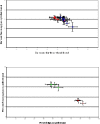
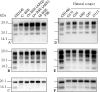
The protease-resistant prion protein (PrP(res)) of a few natural scrapie isolates identified in sheep, reminiscent of the experimental isolate CH1641 derived from a British natural scrapie case, showed partial molecular similarities to ovine bovine spongiform encephalopathy (BSE). Recent discovery of an atypical form of BSE in cattle, L-type BSE or BASE, suggests that also this form of BSE might have been transmitted to sheep. We studied by Western blot the molecular features of PrP(res) in four "CH1641-like" natural scrapie isolates after transmission in an ovine transgenic model (TgOvPrP4), to see if "CH1641-like" isolates might be linked to L-type BSE. We found less diglycosylated PrP(res) than in classical BSE, but similar glycoform proportions and apparent molecular masses of the usual PrP(res) form (PrP(res) #1) to L-type BSE. However, the "CH1641-like" isolates differed from both L-type and classical BSE by an abundant, C-terminally cleaved PrP(res) product (PrP(res) #2) specifically recognised by a C-terminal antibody (SAF84). Differential immunoprecipitation of PrP(res) #1 and PrP(res) #2 resulted in enrichment in PrP(res) #2, and demonstrated the presence of mono- and diglycosylated PrP(res) products. PrP(res) #2 could not be obtained from several experimental scrapie sources (SSBP1, 79A, Chandler, C506M3) in TgOvPrP4 mice, but was identified in the 87V scrapie strain and, in lower and variable proportions, in 5 of 5 natural scrapie isolates with different molecular features to CH1641. PrP(res) #2 identification provides an additional method for the molecular discrimination of prion strains, and demonstrates differences between "CH1641-like" ovine scrapie and bovine L-type BSE transmitted in an ovine transgenic mouse model.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
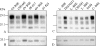





References
-
- Zou WQ, Capellari S, Parchi P, Sy MS, Gambetti P, et al. Identification of novel proteinase K-resistant C-terminal fragments of PrP in Creutzfeldt-Jakob disease. J Biol Chem. 2003;278:40429–40436. - PubMed
-
- Baron T, Madec J-Y, Calavas D, Richard Y, Barillet F. Comparison of French natural scrapie isolates with bovine spongiform encephalopathy and experimental scrapie infected sheep. Neurosci Lett. 2000;284:175–178. - PubMed
-
- Collinge J, Sidle KCL, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of 'new variant' CJD. Nature. 1996;383:685–690. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

