Trogocytosis of MHC-I/peptide complexes derived from tumors and infected cells enhances dendritic cell cross-priming and promotes adaptive T cell responses
- PMID: 18769733
- PMCID: PMC2518214
- DOI: 10.1371/journal.pone.0003097
Trogocytosis of MHC-I/peptide complexes derived from tumors and infected cells enhances dendritic cell cross-priming and promotes adaptive T cell responses
Abstract
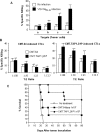
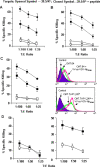
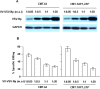
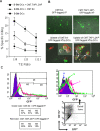
The transporter associated with antigen processing (TAP) and the major histocompatibility complex class I (MHC-I), two important components of the MHC-I antigen presentation pathway, are often deficient in tumor cells. The restoration of their expression has been shown to restore the antigenicity and immunogenicity of tumor cells. However, it is unclear whether TAP and MHC-I expression in tumor cells can affect the induction phase of the T cell response. To address this issue, we expressed viral antigens in tumors that are either deficient or proficient in TAP and MHC-I expression. The relative efficiency of direct immunization or immunization through cross-presentation in promoting adaptive T cell responses was compared. The results demonstrated that stimulation of animals with TAP and MHC-I proficient tumor cells generated antigen specific T cells with greater killing activities than those of TAP and MHC-I deficient tumor cells. This discrepancy was traced to differences in the ability of dendritic cells (DCs) to access and sample different antigen reservoirs in TAP and MHC-I proficient versus deficient cells and thereby stimulate adaptive immune responses through the process of cross-presentation. In addition, our data suggest that the increased activity of T cells is caused by the enhanced DC uptake and utilization of MHC-I/peptide complexes from the proficient cells as an additional source of processed antigen. Furthermore, we demonstrate that immune-escape and metastasis are promoted in the absence of this DC 'arming' mechanism. Physiologically, this novel form of DC antigen sampling resembles trogocytosis, and acts to enhance T cell priming and increase the efficacy of adaptive immune responses against tumors and infectious pathogens.
Conflict of interest statement
Figures



References
-
- Jefferies WA, Kolaitis G, Gabathuler R. IFN-gamma-induced recognition of the antigen-processing variant CMT.64 by cytolytic T cells can be replaced by sequential addition of beta 2 microglobulin and antigenic peptides. J Immunol. 1993;151:2974–2985. - PubMed
-
- Seliger B, Maeurer MJ, Ferrone S. TAP off–tumors on. Immunol Today. 1997;18:292–299. - PubMed
-
- Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;21:455–464. - PubMed
-
- Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol. 2003;195:346–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

