Development of a melting tablet containing promethazine HCl against motion sickness
- PMID: 18770049
- PMCID: PMC2977014
- DOI: 10.1208/s12249-008-9133-x
Development of a melting tablet containing promethazine HCl against motion sickness
Abstract
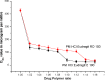
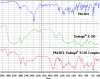
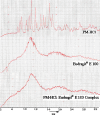
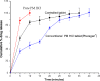
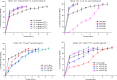
The purpose of this study was to design a 'Traveller Friendly Drug Delivery System' for PM-HCl. Conventional promethazine (PM-HCl) tablets are bitter, need to be taken 1 h before symptoms and water is also needed. Taste-masked granules were produced with Eudragit E100 by extrusion, and analyzed with FTIR, DSC, and XRD. Tablets formulated from granules by direct compression using Ac-Di-Sol, Polyplasdone-XL, Primojel and ion-exchanger Tulsion339 and evaluated for mass uniformity, friability, tensile strength, drug content uniformity, water absorption ratio, in-vitro and in-vivo disintegration time and in-vitro dissolution studies. The observed drug-polymer interactions and reduced crystallinity may be reasons for increased dissolution rates. The formulated tablets were disintegrated within 15 s. Tablets (25 mg PM-HCl) with Ac-Di-Sol (4%) showed complete release within 1 min, while marketed conventional tablets (Phenergan; Rhone-Poulec) release 25% during the same period. A preliminary stability studies for the prepared tablets carried at 30 +/- 2 degrees C/60 +/- 5% RH, and 40 +/- 2 degrees C/75 +/- 5%RH for 3 months showed no significant changes in the tablets quality at 30 +/- 2 degrees C/60 +/- 5% RH. However, at 40 +/- 2 degrees C/75 +/- 5%RH marked increase in in-vitro disintegration time, tensile strength and decrease in friability and water absorption ratio was found. The present studies indicate the abilities of Eudragit E 100 for taste masking and improving the dissolution profile of PM-HCl after complexation. In addition, by employing cost effective direct compression method, fast-dissolving tablets of 400 mg total weight with an acceptable quality could be prepared.
Figures

References
-
- Chang R.-K., Guo X., Burnside B. A., Couch R. A. Fast-dissolving tablets. Pharm. Technol. 2000;24(6):52, 54, 56, 58.
-
- Guidance for Industry Orally Disintegrating Tablet. http://www.fda.gov/cder/guidance/5909dft.htm. Accessed March 3, 2008.
-
- Delgado J. N. Histaminics and Antihistaminics. In: Grisvold W., editor. In Text Book Of Organic Medical and Pharmaceutical Chemistry. 11. Philadelphia: Lippincott; 1998. pp. 710–711.
-
- Bitterness value . European Pharmacopoeia. 5. Strasbourg: Council of Europe; 2004. pp. 221–223.
-
- GmbH R. Eudragit®: Polymethacrylate for phatmaceutical use, Technical literature. Darmstadt, Germany.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

