Comparison of the antiviral activity of adefovir and tenofovir on hepatitis B virus in HIV-HBV-coinfected patients
- PMID: 18771054
- PMCID: PMC2665195
Comparison of the antiviral activity of adefovir and tenofovir on hepatitis B virus in HIV-HBV-coinfected patients
Abstract
Background: Characteristics and factors influencing viral decay under tenofovir (TDF) and adefovir (ADV) need to be determined in HIV-HBV-coinfected patients.
Methods: This open-label study compared the HBV dynamics in 85 HIV-HBV-coinfected patients initiating an antiretroviral regimen, either including TDF or associated with ADV. The first 6-month change in viral load was analysed using mixed linear models. The adjusted hazards ratio, comparing the rates of undetectable HBV DNA between treatments, was calculated using a Cox proportional hazard model.
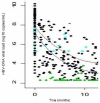
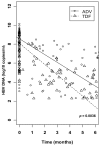
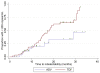
Results: The HBV DNA decay, adjusted for baseline HBV viral load was more pronounced in patients treated with TDF than with ADV at 12 months (66% versus 53%, P=0.0001). Patients in the TDF group presented a steeper slope of decline at 1.1 (95% confidence interval [CI] 0.9-1.3), compared with 0.8 (95% CI 0.6-1.0) in the ADV group (P=0.036). The mean time to HBV DNA undetectability was 19.3 months (95% CI 16.7-22.0) with TDF and 25.9 months (95% CI 21.1-30.7) with ADV. When adjusted for hepatitis B virus e antigen, HBV DNA and alanine aminotransferase levels at baseline, the influence of treatment on time to HBV DNA undetectability remained in favour of TDF versus ADV (hazard ratio=2.79, 95% CI 1.05-7.40, P=0.039)
Conclusions: TDF influenced more strongly the early-phase HBV DNA kinetics than ADV. This is associated with a sustained antiviral activity in the TDF group, in which patients reached the threshold of HBV undetectability at a faster rate and in a larger proportion than those taking ADV.
Figures
Similar articles
-
Tenofovir vs lamivudine plus adefovir in chronic hepatitis B: TENOSIMP-B study.World J Gastroenterol. 2017 Nov 7;23(41):7459-7469. doi: 10.3748/wjg.v23.i41.7459. World J Gastroenterol. 2017. PMID: 29151700 Free PMC article. Clinical Trial.
-
Lamivudine plus tenofovir versus lamivudine plus adefovir for the treatment of hepatitis B virus in HIV-coinfected patients, starting antiretroviral therapy.Indian J Med Microbiol. 2018 Apr-Jun;36(2):217-223. doi: 10.4103/ijmm.IJMM_17_37. Indian J Med Microbiol. 2018. PMID: 30084414 Clinical Trial.
-
Hepatitis B virus wild-type and rtN236T populations show similar early HBV DNA decline in adefovir refractory patients on a tenofovir-based regimen.J Viral Hepat. 2013 Feb;20(2):131-40. doi: 10.1111/j.1365-2893.2012.01638.x. Epub 2012 Jul 9. J Viral Hepat. 2013. PMID: 23301548
-
Virology and clinical sequelae of drug-resistant HBV in HIV-HBV-coinfected patients on highly active antiretroviral therapy.Antivir Ther. 2010;15(3 Pt B):487-91. doi: 10.3851/IMP1553. Antivir Ther. 2010. PMID: 20516569 Free PMC article. Review.
-
Evaluating the Efficacy of Repurposed Antiretrovirals in Hepatitis B Virus Treatment: A Narrative Review of the Pros and Cons.Int J Mol Sci. 2025 Jan 23;26(3):925. doi: 10.3390/ijms26030925. Int J Mol Sci. 2025. PMID: 39940695 Free PMC article. Review.
Cited by
-
Antiviral treatment of chronic hepatitis B virus (HBV) infections.Viruses. 2010 Jun;2(6):1279-1305. doi: 10.3390/v2061279. Epub 2010 May 31. Viruses. 2010. PMID: 21994680 Free PMC article.
-
Comparative effectiveness of anti-viral drugs with dual activity for treating hepatitis B and HIV co-infected patients: a network meta-analysis.BMC Infect Dis. 2018 Nov 14;18(1):564. doi: 10.1186/s12879-018-3506-x. BMC Infect Dis. 2018. PMID: 30428847 Free PMC article.
-
Comparison of the efficacy of tenofovir and adefovir in the treatment of chronic hepatitis B: a systematic review.Virol J. 2011 Mar 9;8:111. doi: 10.1186/1743-422X-8-111. Virol J. 2011. PMID: 21388525 Free PMC article.
-
Efficacy and safety of tenofovir disoproxil fumarate in patients with chronic hepatitis B.Therap Adv Gastroenterol. 2010 Mar;3(2):107-19. doi: 10.1177/1756283X09354562. Therap Adv Gastroenterol. 2010. PMID: 21180595 Free PMC article.
-
Hepatitis B in HIV: available treatment options and approach to therapy.Curr Infect Dis Rep. 2009 Sep;11(5):407-13. doi: 10.1007/s11908-009-0057-8. Curr Infect Dis Rep. 2009. PMID: 19698285
References
-
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. Journal of Viral Hepatitis. 2004;11:97–107. - PubMed
-
- Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593–601. - PubMed
-
- Bodsworth N, Cooper D, Donovan B. The influence of human immunodeficiency virus type 1 infection on the development of the hepatitis B virus carrier state. J Infect Dis. 1991;163:1138–1140. - PubMed
-
- Krogsgaard K, Bindslev N, Christensen E, et al. The treatment effect of alpha interferon in chronic hepatitis B is independent of pre-treatment variables. Results based on individual patient data from 10 clinical controlled trials. European Concerted Action on Viral Hepatitis (Eurohep) J Hepatol. 1994;21:646–655. - PubMed
-
- Marcellin P, Lau GKK, Bonino F, et al. Peginterferon Alfa-2a Alone, Lamivudine Alone, and the Two in Combination in Patients with HBeAg-Negative Chronic Hepatitis B. N Engl J Med. 2004;351:1206–1217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
