Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins
- PMID: 18771283
- PMCID: PMC3045568
- DOI: 10.1021/bi8011862
Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins
Abstract
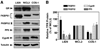
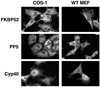
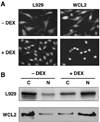
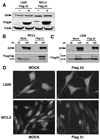
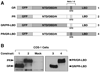
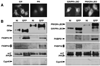
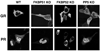
The TPR proteins FKBP52, FKBP51, Cyp40, and PP5 are found in steroid receptor (SR) complexes, but their receptor-specific preferences and roles remain unresolved. We have undertaken a systematic approach to this problem by examining the contribution of all four TPRs to the localization properties of glucocorticoid (GR) and progesterone (PR) receptors. The GR of L929 cells was found in the cytoplasm in a complex containing PP5 and FKBP51, while the GR of WCL2 cells was nuclear and contained PP5 and FKBP52. Cyp40 did not interact with the GR in either cell line. To test whether FKBP interaction determined localization, we overexpressed Flag-tagged FKBP51 in WCL2 cells and Flag-FKBP52 in L929 cells. In WCL2 cells, the GR exhibited a shift to greater cytoplasmic localization that correlated with recruitment of Flag-FKBP51. In contrast, Flag-FKBP52 was not recruited to the GR of L929 cells, and no change in localization was observed, suggesting that both cell-type-specific mechanisms and TPR abundance contribute to the SR-TPR interaction. As a further test, GR-GFP and PR-GFP constructs were expressed in COS cells. The GR-GFP construct localized to the cytoplasm, while the PR-GFP construct was predominantly nuclear. Similar to L929 cells, the GR in COS interacted with PP5 and FKBP51, while PR interacted with FKBP52. Analysis of GR-PR chimeric constructs revealed that the ligand-binding domain of each receptor determines both TPR specificity and localization. Lastly, we analyzed GR and PR localization in cells completely lacking TPR. PR in FKBP52 KO cells showed a complete shift to the cytoplasm, while GR in FKBP51 KO and PP5 KO cells showed a moderate shift to the nucleus, indicating that both TPRs contribute to GR localization. Our results demonstrate that SRs have distinct preferences for TPR proteins, a property that resides in the LBD and which can now explain long-standing differences in receptor subcellular localization.
Figures
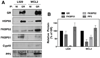

Similar articles
-
Differential control of glucocorticoid receptor hormone-binding function by tetratricopeptide repeat (TPR) proteins and the immunosuppressive ligand FK506.Biochemistry. 2005 Feb 15;44(6):2030-8. doi: 10.1021/bi048503v. Biochemistry. 2005. PMID: 15697228
-
Separable features of the ligand-binding domain determine the differential subcellular localization and ligand-binding specificity of glucocorticoid receptor and progesterone receptor.Mol Endocrinol. 2001 Jan;15(1):17-31. doi: 10.1210/mend.15.1.0584. Mol Endocrinol. 2001. PMID: 11145736
-
Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes.Mol Endocrinol. 1998 Mar;12(3):342-54. doi: 10.1210/mend.12.3.0075. Mol Endocrinol. 1998. PMID: 9514152
-
Therapeutic Targeting of the FKBP52 Co-Chaperone in Steroid Hormone Receptor-Regulated Physiology and Disease.Curr Mol Pharmacol. 2015;9(2):109-25. doi: 10.2174/1874467208666150519114115. Curr Mol Pharmacol. 2015. PMID: 25986565 Review.
-
Regulation of steroid hormone receptor function by the 52-kDa FK506-binding protein (FKBP52).Curr Opin Pharmacol. 2011 Aug;11(4):314-9. doi: 10.1016/j.coph.2011.03.010. Epub 2011 Apr 19. Curr Opin Pharmacol. 2011. PMID: 21511531 Free PMC article. Review.
Cited by
-
Alterations in the steroid hormone receptor co-chaperone FKBPL are associated with male infertility: a case-control study.Reprod Biol Endocrinol. 2010 Mar 8;8:22. doi: 10.1186/1477-7827-8-22. Reprod Biol Endocrinol. 2010. PMID: 20210997 Free PMC article.
-
Protein phosphatase 5 mediates lipid metabolism through reciprocal control of glucocorticoid receptor and peroxisome proliferator-activated receptor-γ (PPARγ).J Biol Chem. 2011 Dec 16;286(50):42911-22. doi: 10.1074/jbc.M111.311662. Epub 2011 Oct 12. J Biol Chem. 2011. PMID: 21994940 Free PMC article.
-
Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta.Mol Cell Biol. 2009 Sep;29(17):4788-97. doi: 10.1128/MCB.00649-09. Epub 2009 Jul 6. Mol Cell Biol. 2009. PMID: 19581287 Free PMC article.
-
Combined x-ray crystallography and computational modeling approach to investigate the Hsp90 C-terminal peptide binding to FKBP51.Sci Rep. 2017 Oct 27;7(1):14288. doi: 10.1038/s41598-017-14731-z. Sci Rep. 2017. PMID: 29079741 Free PMC article.
-
Steroid Receptor-Associated Immunophilins: A Gateway to Steroid Signalling.Clin Biochem Rev. 2015 May;36(2):31-52. Clin Biochem Rev. 2015. PMID: 26224894 Free PMC article. Review.
References
-
- Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6(3):225–236. - PubMed
-
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18(3):306–360. - PubMed
-
- Pratt WB, Galigniana MD, Harrell JM, DeFranco DB. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal. 2004;16(8):857–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

