Glycoengineering of human IgG1-Fc through combined yeast expression and in vitro chemoenzymatic glycosylation
- PMID: 18771295
- PMCID: PMC2628294
- DOI: 10.1021/bi800874y
Glycoengineering of human IgG1-Fc through combined yeast expression and in vitro chemoenzymatic glycosylation
Abstract
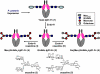
The presence and precise structures of the glycans attached at the Fc domain of monoclonal antibodies play an important role in determining antibodies' effector functions such as antibody-dependent cell cytotoxicity (ADCC), complement activation, and anti-inflammatory activity. This paper describes a novel approach for glycoengineering of human IgG1-Fc that combines high-yield expression of human IgG1-Fc in yeast and subsequent in vitro enzymatic glycosylation, using the endoglycosidase-catalyzed transglycosylation as the key reaction. Human IgG1-Fc was first overproduced in Pichia pastoris. Then the heterogeneous yeast glycans were removed by Endo-H treatment to give the GlcNAc-containing IgG1-Fc as a homodimer. Finally, selected homogeneous glycans were attached to the GlcNAc-primer in the IgG1-Fc through an endoglycosidase-catalyzed transglycosylation, using sugar oxazolines as the donor substrates. It was found that the enzymatic transglycosylation was efficient with native GlcNAc-containing IgG1-Fc homodimer without the need to denature the protein, and the reaction could proceed to completion to give homogeneous glycoforms of IgG1-Fc when an excess of oligosaccharide oxazolines was used as the donor substrates. The binding of the synthetic IgG1-Fc glycoforms to the FcgammaIIIa receptor was also investigated. This novel glycoengineering approach should be useful for providing various homogeneous, natural or synthetic glycoforms of IgG1-Fc for structure-function relationship studies, and for future clinical applications.
Figures
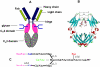





Similar articles
-
Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions.J Am Chem Soc. 2012 Jul 25;134(29):12308-18. doi: 10.1021/ja3051266. Epub 2012 Jul 16. J Am Chem Soc. 2012. PMID: 22747414 Free PMC article.
-
Influence of N-glycosylation on effector functions and thermal stability of glycoengineered IgG1 monoclonal antibody with homogeneous glycoforms.MAbs. 2019 Feb/Mar;11(2):350-372. doi: 10.1080/19420862.2018.1551044. Epub 2018 Dec 10. MAbs. 2019. PMID: 30466347 Free PMC article.
-
Chemoenzymatic synthesis and Fcγ receptor binding of homogeneous glycoforms of antibody Fc domain. Presence of a bisecting sugar moiety enhances the affinity of Fc to FcγIIIa receptor.J Am Chem Soc. 2011 Nov 23;133(46):18975-91. doi: 10.1021/ja208390n. Epub 2011 Nov 1. J Am Chem Soc. 2011. PMID: 22004528 Free PMC article.
-
Glycosylation engineering of therapeutic IgG antibodies: challenges for the safety, functionality and efficacy.Protein Cell. 2018 Jan;9(1):47-62. doi: 10.1007/s13238-017-0433-3. Epub 2017 Jun 8. Protein Cell. 2018. PMID: 28597152 Free PMC article. Review.
-
Crystallizable Fragment Glycoengineering for Therapeutic Antibodies Development.Front Immunol. 2017 Nov 13;8:1554. doi: 10.3389/fimmu.2017.01554. eCollection 2017. Front Immunol. 2017. PMID: 29181010 Free PMC article. Review.
Cited by
-
Intramolecular N-glycan/polypeptide interactions observed at multiple N-glycan remodeling steps through [(13)C,(15)N]-N-acetylglucosamine labeling of immunoglobulin G1.Biochemistry. 2015 Jan 20;54(2):313-22. doi: 10.1021/bi501380t. Epub 2014 Dec 31. Biochemistry. 2015. PMID: 25551295 Free PMC article.
-
Arthrobacter endo-beta-N-acetylglucosaminidase shows transglycosylation activity on complex-type N-glycan oxazolines: one-pot conversion of ribonuclease B to sialylated ribonuclease C.Chembiochem. 2010 Jul 5;11(10):1350-5. doi: 10.1002/cbic.201000242. Chembiochem. 2010. PMID: 20486148 Free PMC article.
-
Innovative Preparation of Biopharmaceuticals Using Transglycosylation Activity of Microbial Endoglycosidases.J Appl Glycosci (1999). 2021 Mar 11;68(1):1-9. doi: 10.5458/jag.jag.JAG-2020_0013. eCollection 2021. J Appl Glycosci (1999). 2021. PMID: 34354540 Free PMC article.
-
Enzymatic transglycosylation for glycoconjugate synthesis.Curr Opin Chem Biol. 2009 Dec;13(5-6):592-600. doi: 10.1016/j.cbpa.2009.08.014. Epub 2009 Sep 18. Curr Opin Chem Biol. 2009. PMID: 19766528 Free PMC article. Review.
-
Discovery of a single-subunit oligosaccharyltransferase that enables glycosylation of full-length IgG antibodies in bacteria.Nat Commun. 2025 Jul 4;16(1):6152. doi: 10.1038/s41467-025-61440-7. Nat Commun. 2025. PMID: 40610439 Free PMC article.
References
-
- Dillman RO. Monoclonal antibodies in the treatment of malignancy: basic concepts and recent developments. Cancer Invest. 2001;19:833–841. - PubMed
-
- Schaedel O, Reiter Y. Antibodies and their fragments as anti-cancer agents. Curr. Pharm. Des. 2006;12:363–378. - PubMed
-
- Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert Opin. Biol. Ther. 2007;7:1401–1413. - PubMed
-
- Strome SE, Sausville EA, Mann D. A mechanistic perspective of monoclonal antibodies in cancer therapy beyond target-related effects. Oncologist. 2007;12:1084–1095. - PubMed
-
- Jefferis R. Glycosylation of recombinant antibody therapeutics. Biotechnol. Prog. 2005;21:11–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

