Identification of the most active interleukin-32 isoform
- PMID: 18771438
- PMCID: PMC2673365
- DOI: 10.1111/j.1365-2567.2008.02917.x
Identification of the most active interleukin-32 isoform
Abstract
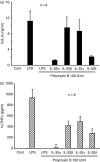
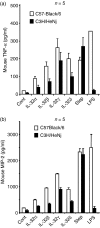
Cytokines are crucial in host defence against pathogens such as bacteria, viruses, fungi and parasites. A newly described cytokine, interleukin-32 (IL-32), induces various proinflammatory cytokines (tumour necrosis factor-alpha, IL-1beta, IL-6) and chemokines in both human and mouse cells through the nuclear factor-kappaB and p38 mitogen-activated protein kinase inflammatory signal pathway. The IL-32 primarily acts on monocytic cells rather than T cells. In an attempt to isolate the IL-32 soluble receptor, we used an IL-32 ligand-affinity column to purify neutrophil proteinase 3, which is a serine proteinase involved in many inflammatory diseases. IL-32 has biological activity associated with Mycobacterium tuberculosis and chronic proinflammatory diseases such as rheumatoid arthritis. IL-32 is transcribed as six alternative splice variants and the biological activity of each individual isoform remains unknown. Here, we cloned the complementary DNA of the four IL-32 isoforms (alpha, beta, gamma and delta) that are the most representative IL-32 transcripts. To produce recombinant protein with a high yield, the amino acids of two cysteine residues were mutated to serine residues, because serine residues are not conserved among different species. The multi-step purified recombinant IL-32 isoform proteins were assessed for their biological activities with different cytokine assays. The gamma isoform of IL-32 was the most active, although all isoforms were biologically active. The present study will provide a specific target to neutralize endogenous IL-32, which may contribute to basic and clinical immunology.
Figures




Similar articles
-
Interleukin-32 gamma specific monoclonal antibody and developing IL-32 specific ELISA.Hybridoma (Larchmt). 2010 Dec;29(6):501-9. doi: 10.1089/hyb.2010.0059. Epub 2010 Nov 18. Hybridoma (Larchmt). 2010. PMID: 21087097
-
In vitro activation of murine peritoneal macrophages by ultraviolet B radiation: upregulation of CD18, production of NO, proinflammatory cytokines and a signal transduction pathway.Mol Immunol. 2004 Apr;40(18):1315-23. doi: 10.1016/j.molimm.2004.01.001. Mol Immunol. 2004. PMID: 15072850
-
Identification of constitutively active interleukin 33 (IL-33) splice variant.J Biol Chem. 2011 Jun 3;286(22):20078-86. doi: 10.1074/jbc.M111.219089. Epub 2011 Mar 22. J Biol Chem. 2011. PMID: 21454686 Free PMC article.
-
IL-32, a novel cytokine with a possible role in disease.Ann Rheum Dis. 2006 Nov;65 Suppl 3(Suppl 3):iii61-4. doi: 10.1136/ard.2006.058511. Ann Rheum Dis. 2006. PMID: 17038476 Free PMC article. Review.
-
Novel insights into the biology of interleukin-32.Cell Mol Life Sci. 2013 Oct;70(20):3883-92. doi: 10.1007/s00018-013-1301-9. Epub 2013 Mar 6. Cell Mol Life Sci. 2013. PMID: 23463238 Free PMC article. Review.
Cited by
-
Oxidative stress induced interleukin-32 mRNA expression in human bronchial epithelial cells.Respir Res. 2012 Mar 14;13(1):19. doi: 10.1186/1465-9921-13-19. Respir Res. 2012. PMID: 22413812 Free PMC article.
-
Innate immunity triggers IL-32 expression by fibroblast-like synoviocytes in rheumatoid arthritis.Arthritis Res Ther. 2010;12(4):R135. doi: 10.1186/ar3073. Epub 2010 Jul 8. Arthritis Res Ther. 2010. PMID: 20615213 Free PMC article.
-
Cytokines in the pathogenesis of rheumatoid arthritis: new players and therapeutic targets.BMC Rheumatol. 2017 Nov 28;1:3. doi: 10.1186/s41927-017-0001-8. eCollection 2017. BMC Rheumatol. 2017. PMID: 30886947 Free PMC article. Review.
-
Interleukin-32γ in the Control of Acute Experimental Chagas Disease.J Immunol Res. 2022 Jan 20;2022:7070301. doi: 10.1155/2022/7070301. eCollection 2022. J Immunol Res. 2022. PMID: 35097133 Free PMC article.
-
The Role of Pro-Inflammatory Mediator Interleukin-32 in Osteoclast Differentiation.Turk J Pharm Sci. 2023 May 9;20(2):121-125. doi: 10.4274/tjps.galenos.2022.69922. Turk J Pharm Sci. 2023. PMID: 37161688 Free PMC article.
References
-
- Bombardieri M, McInnes IB, Pitzalis C. Interleukin-18 as a potential therapeutic target in chronic autoimmune/inflammatory conditions. Expert Opin Biol Ther. 2007;7:31–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

