Signalling mechanisms regulating the activation of human eosinophils by mast-cell-derived chymase: implications for mast cell-eosinophil interaction in allergic inflammation
- PMID: 18771439
- PMCID: PMC2673369
- DOI: 10.1111/j.1365-2567.2008.02916.x
Signalling mechanisms regulating the activation of human eosinophils by mast-cell-derived chymase: implications for mast cell-eosinophil interaction in allergic inflammation
Abstract
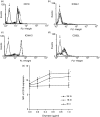
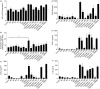
Allergic diseases such as asthma and allergic dermatitis are associated with the degranulation of mast cells. Chymase, a mast-cell-specific protease, is the major component in mast cell granules that can induce eosinophil infiltration into inflammatory sites. We examined the immunopathological mechanisms for the activation of eosinophils by chymase in allergic inflammation. Cytokines were measured by cytometric bead array Flex Sets multiplex assay using flow cytometry and enzyme-linked immunosorbent assay. Adhesion molecules, migration and intracellular signalling pathways were assessed by flow cytometry, Boyden chamber assay and Western blot, respectively. Chymase suppressed the apoptosis of eosinophils and induce the release of the cytokine interleukin-6 (IL-6) and chemokines CXCL8, CCL2 and CXCL1 by eosinophils dose-dependently. It also up-regulated the surface expression of adhesion molecule CD18 and stimulated the chemokinetic migration of eosinophils. The expressions of adhesion molecules, cytokines and chemokines, and chemokinetic migration were differentially regulated by the activation of extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, Akt, Janus-activated kinase and nuclear factor-kappaB pathways. Chymase therefore plays a pivotal immunological role in the interaction between mast cells and eosinophils in allergic diseases such as allergic dermatitis by inducing adhesion molecule-mediated chemokinetic migration and inflammatory cytokines and chemokines of eosinophils, through multiple intracellular signalling molecules and transcription factor. Our results therefore provide a further biochemical basis for the pathogenesis of allergic inflammation consequent on the interaction between mast cells and eosinophils, and give insight for the development of new therapies.
Figures



Similar articles
-
Leptin-mediated cytokine release and migration of eosinophils: implications for immunopathophysiology of allergic inflammation.Eur J Immunol. 2007 Aug;37(8):2337-48. doi: 10.1002/eji.200636866. Eur J Immunol. 2007. PMID: 17634954
-
Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation.Am J Respir Cell Mol Biol. 2010 Sep;43(3):305-15. doi: 10.1165/rcmb.2009-0168OC. Epub 2009 Oct 20. Am J Respir Cell Mol Biol. 2010. PMID: 19843704
-
Intracellular signaling mechanisms regulating the activation of human eosinophils by the novel Th2 cytokine IL-33: implications for allergic inflammation.Cell Mol Immunol. 2010 Jan;7(1):26-34. doi: 10.1038/cmi.2009.106. Epub 2009 Dec 23. Cell Mol Immunol. 2010. PMID: 20029461 Free PMC article.
-
The role of human mast cell-derived cytokines in eosinophil biology.J Interferon Cytokine Res. 2004 May;24(5):271-81. doi: 10.1089/107999004323065057. J Interferon Cytokine Res. 2004. PMID: 15153310 Review.
-
Biochemical assessment of intracellular signal transduction pathways in eosinophils: implications for pharmacotherapy.Crit Rev Clin Lab Sci. 2004;41(1):79-113. doi: 10.1080/10408360490427624. Crit Rev Clin Lab Sci. 2004. PMID: 15077724 Review.
Cited by
-
Eosinophils as Major Player in Type 2 Inflammation: Autoimmunity and Beyond.Adv Exp Med Biol. 2021;1347:197-219. doi: 10.1007/5584_2021_640. Adv Exp Med Biol. 2021. PMID: 34031864 Review.
-
IL33: Roles in Allergic Inflammation and Therapeutic Perspectives.Front Immunol. 2019 Mar 4;10:364. doi: 10.3389/fimmu.2019.00364. eCollection 2019. Front Immunol. 2019. PMID: 30886621 Free PMC article. Review.
-
Induction of mast cell accumulation by chymase via an enzymatic activity- and intercellular adhesion molecule-1-dependent mechanism.Br J Pharmacol. 2018 Feb;175(4):678-692. doi: 10.1111/bph.14117. Epub 2018 Jan 18. Br J Pharmacol. 2018. PMID: 29197072 Free PMC article.
-
Effects of Lidocaine-Derived Organic Compounds on Eosinophil Activation and Survival.Molecules. 2023 Jul 27;28(15):5696. doi: 10.3390/molecules28155696. Molecules. 2023. PMID: 37570665 Free PMC article.
-
Bovine Immune Factors Underlying Tick Resistance: Integration and Future Directions.Front Cell Infect Microbiol. 2017 Dec 19;7:522. doi: 10.3389/fcimb.2017.00522. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29312898 Free PMC article. Review.
References
-
- Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2006;117:S450–6. - PubMed
-
- Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–84. - PubMed
-
- Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, Bradding P. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med. 2005;171:1103–8. - PubMed
-
- Kinoshita M, Okada M, Hara M, Furukawa Y, Matsumori A. Mast cell tryptase in mast cell granules enhances MCP-1 and interleukin-8 production in human endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:1858–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

