Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain
- PMID: 18771689
- PMCID: PMC3216633
- DOI: 10.1016/j.neuro.2008.08.002
Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain
Abstract
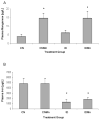
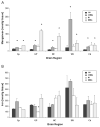
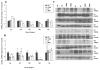
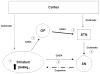
Unlike other essential trace elements (e.g., zinc and iron) it is the toxicity of manganese (Mn) that is more common in human populations than its deficiency. Data suggest alterations in dopamine biology may drive the effects associated with Mn neurotoxicity, though recently gamma-aminobutyric acid (GABA) has been implicated. In addition, iron deficiency (ID), a common nutritional problem, may cause disturbances in neurochemistry by facilitating accumulation of Mn in the brain. Previous data from our lab have shown decreased brain tissue levels of GABA as well as decreased (3)H-GABA uptake in synaptosomes as a result of Mn exposure and ID. These results indicate a possible increase in the concentration of extracellular GABA due to alterations in expression of GABA transport and receptor proteins. In this study weanling-male Sprague-Dawley rats were randomly placed into one of four dietary treatment groups: control (CN; 35mg Fe/kg diet), iron-deficient (ID; 6mg Fe/kg diet), CN with Mn supplementation (via the drinking water; 1g Mn/l) (CNMn), and ID with Mn supplementation (IDMn). Using in vivo microdialysis, an increase in extracellular GABA concentrations in the striatum was observed in response to Mn exposure and ID although correlational analysis reveals that extracellular GABA is related more to extracellular iron levels and not Mn. A diverse effect of Mn exposure and ID was observed in the regions examined via Western blot and RT-PCR analysis, with effects on mRNA and protein expression of GAT-1, GABA(A), and GABA(B) differing between and within the regions examined. For example, Mn exposure reduced GAT-1 protein expression by approximately 50% in the substantia nigra, while increasing mRNA expression approximately four-fold, while in the caudate putamen mRNA expression was decreased with no effect on protein expression. These data suggest that Mn exposure results in an increase in extracellular GABA concentrations via altered expression of transport and receptor proteins, which may be the basis of the neurological characteristics of manganism.
Figures



References
-
- Anderson JG, Cooney PT, Erikson KM. Brain manganese accumulation is inversely related to GABA uptake in male and female rats. Tox Sci. 2007a;95:188–95. - PubMed
-
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Tox. 2005;35:1–32. - PubMed
-
- Bae JH, Jang BC, Suh SI, Ha E, Baik HH, Kim SS, Lee MY, Shin DH. Manganese induces inducible nitric oxide synthase (iNOS) expression via activation of both MAP kinase and PI3K/Akt pathways in BV2 microglial cells. Neurosci Lett. 2006;398:151–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

