Co-occurrence of Alzheimer's disease ß-amyloid and τ pathologies at synapses
- PMID: 18771816
- PMCID: PMC2909664
- DOI: 10.1016/j.neurobiolaging.2008.07.021
Co-occurrence of Alzheimer's disease ß-amyloid and τ pathologies at synapses
Abstract
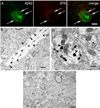
Although beta-amyloid (Abeta) plaques and tau neurofibrillary tangles are hallmarks of Alzheimer's disease (AD) neuropathology, loss of synapses is considered the best correlate of cognitive decline in AD, rather than plaques or tangles. How pathological Abeta and tau aggregation relate to each other and to alterations in synapses remains unclear. Since aberrant tau phosphorylation occurs in amyloid precursor protein (APP) Swedish mutant transgenic mice, and since neurofibrillary tangles develop in triple transgenic mice harboring mutations in APP, tau and presenilin 1, we utilized these well-characterized mouse models to explore the relation between Abeta and tau pathologies. We now report that pathological accumulation of Abeta and hyperphosphorylation of tau develop concomitantly within synaptic terminals.
Copyright 2008 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors disclose that there are no actual or potential conflicts of interest including any financial, personal or other relationship with other people or organizations.
Figures


Similar articles
-
Familial Alzheimer's disease mutations at position 22 of the amyloid β-peptide sequence differentially affect synaptic loss, tau phosphorylation and neuronal cell death in an ex vivo system.PLoS One. 2020 Sep 23;15(9):e0239584. doi: 10.1371/journal.pone.0239584. eCollection 2020. PLoS One. 2020. PMID: 32966331 Free PMC article.
-
Human tau increases amyloid β plaque size but not amyloid β-mediated synapse loss in a novel mouse model of Alzheimer's disease.Eur J Neurosci. 2016 Dec;44(12):3056-3066. doi: 10.1111/ejn.13442. Epub 2016 Nov 12. Eur J Neurosci. 2016. PMID: 27748574 Free PMC article.
-
Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline.J Neurosci. 2010 May 26;30(21):7281-9. doi: 10.1523/JNEUROSCI.0490-10.2010. J Neurosci. 2010. PMID: 20505094 Free PMC article.
-
Amyloid-induced neurofibrillary tangle formation in Alzheimer's disease: insight from transgenic mouse and tissue-culture models.Int J Dev Neurosci. 2004 Nov;22(7):453-65. doi: 10.1016/j.ijdevneu.2004.07.013. Int J Dev Neurosci. 2004. PMID: 15465275 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Conductance-Based Structural Brain Connectivity in Aging and Dementia.Brain Connect. 2021 Sep;11(7):566-583. doi: 10.1089/brain.2020.0903. Epub 2021 May 27. Brain Connect. 2021. PMID: 34042511 Free PMC article.
-
Critical role of intraneuronal Aβ in Alzheimer's disease: technical challenges in studying intracellular Aβ.Life Sci. 2012 Dec 10;91(23-24):1153-8. doi: 10.1016/j.lfs.2012.06.004. Epub 2012 Jun 19. Life Sci. 2012. PMID: 22727791 Free PMC article.
-
Oxidative stress and hippocampal synaptic protein levels in elderly cognitively intact individuals with Alzheimer's disease pathology.Neurobiol Aging. 2016 Jun;42:1-12. doi: 10.1016/j.neurobiolaging.2016.02.030. Epub 2016 Mar 8. Neurobiol Aging. 2016. PMID: 27143416 Free PMC article.
-
Alzheimer's disease: a clinical practice-oriented review.Front Neurol. 2012 Apr 20;3:63. doi: 10.3389/fneur.2012.00063. eCollection 2012. Front Neurol. 2012. PMID: 22529838 Free PMC article.
-
Synaptic Elimination in Neurological Disorders.Curr Neuropharmacol. 2019;17(11):1071-1095. doi: 10.2174/1570159X17666190603170511. Curr Neuropharmacol. 2019. PMID: 31161981 Free PMC article. Review.
References
-
- Alafuzoff I, Pikkarainen M, Arzberger T, Thal DR, Al-Sarraj S, Bell J, Bodi I, Budka H, Capetillo-Zarate E, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kavantzas N, King A, Korkolopoulou P, Kovacs GG, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Stadelmann C, Streichenberger N, Tagliavini F, Kretzschmar H. Inter-laboratory comparison of neuropathological assessments of beta-amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol. 2008;115:533–546. - PubMed
-
- Bayer TA, Wirths O. Review on the APP/PS1KI mouse model: intraneuronal Abeta accumulation triggers axonopathy, neuron loss and working memory impairment. Genes. Brain Behav. 2008;7 Suppl 1:6–11. - PubMed
-
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. - PubMed
-
- Busciglio J, Pelsman A, Wong C, Pigino G, Yuan M, Mori H, Yankner BA. Altered metabolism of the amyloid beta precursor protein is associated with mitochondrial dysfunction in Down's syndrome. Neuron. 2002;33:677–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

