Large ventral lateral neurons modulate arousal and sleep in Drosophila
- PMID: 18771923
- PMCID: PMC2597195
- DOI: 10.1016/j.cub.2008.08.033
Large ventral lateral neurons modulate arousal and sleep in Drosophila
Abstract
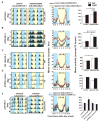
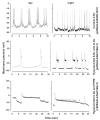
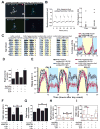
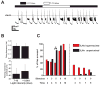
Background: Large ventral lateral clock neurons (lLNvs) exhibit higher daytime-light-driven spontaneous action-potential firing rates in Drosophila, coinciding with wakefulness and locomotor-activity behavior. To determine whether the lLNvs are involved in arousal and sleep/wake behavior, we examined the effects of altered electrical excitation of the LNvs.
Results: LNv-hyperexcited flies reverse the normal day-night firing pattern, showing higher lLNv firing rates at night and pigment-dispersing-factor-mediated enhancement of nocturnal locomotor-activity behavior and reduced quantity and quality of sleep. lLNv hyperexcitation impairs sensory arousal, as shown by physiological and behavioral assays. lLNv-hyperexcited flies lacking sLNvs exhibit robust hyperexcitation-induced increases in nocturnal behavior, suggesting that the sLNvs are not essential for mediation of arousal.
Conclusions: Light-activated lLNvs modulate behavioral arousal and sleep in Drosophila.
Figures


References
-
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nature Reviews. 2002;3:591–605. - PubMed
-
- Holmes TC, Sheeba V, Mizrak D, Rubovszky B, Dahdal D. Circuit-breaking and behavioral analysis by molecular genetic manipulation of neural activity in Drosophila. In: North G, Greenspan R, editors. Invertebrate Neurobiology. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2007. pp. 19–52.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

