New insights on mast cell activation via the high affinity receptor for IgE
- PMID: 18772004
- PMCID: PMC2761150
- DOI: 10.1016/S0065-2776(08)00403-3
New insights on mast cell activation via the high affinity receptor for IgE
Abstract
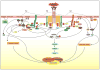
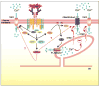
Mast cells are innate immune cells that function as regulatory or effector cells and serve to amplify adaptive immunity. In adaptive immunity these cells function primarily through cell surface Fc receptors that bind immunoglobulin antibodies. The dysregulation of their adaptive role makes them central players in allergy and asthma. Upon encountering an allergen (antigen), which is recognized by immunoglobulin E (IgE) antibodies bound to the high affinity IgE receptor (FcepsilonRI) expressed on their cell surface, mast cells secrete both preformed and newly synthesized mediators of the allergic response. Blocking of these responses is an objective in therapeutic intervention of allergic diseases. Thus, understanding the mechanisms by which antigens elicit mast cell activation (via FcepsilonRI) holds promise toward identifying therapeutic targets. Here we review the most recent advances in understanding antigen-dependent mast cell activation. Specifically, we focus on the requirements for FcepsilonRI activation, the regulation of calcium responses, co-stimulatory signals in FcepsilonRI-mediated mast cell activation and function, and how genetics influences mast cell signaling and responses. These recent discoveries open new avenues of investigation with therapeutic potential.
Figures

References
-
- Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110δ phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. - PubMed
-
- Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582:222–227. - PubMed
-
- Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. - PubMed
-
- Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, Kane SA, Peters SP, Penn RB, Spiegel S, Panettieri RA., Jr Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. Faseb J. 2001;15:1212–1214. - PubMed
-
- Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

