Calcification of multipotent prostate tumor endothelium
- PMID: 18772110
- PMCID: PMC2604136
- DOI: 10.1016/j.ccr.2008.06.017
Calcification of multipotent prostate tumor endothelium
Abstract
Solid tumors require new blood vessels for growth and metastasis, yet the biology of tumor-specific endothelial cells is poorly understood. We have isolated tumor endothelial cells from mice that spontaneously develop prostate tumors. Clonal populations of tumor endothelial cells expressed hematopoietic and mesenchymal stem cell markers and differentiated to form cartilage- and bone-like tissues. Chondrogenic differentiation was accompanied by an upregulation of cartilage-specific col2a1 and sox9, whereas osteocalcin and the metastasis marker osteopontin were upregulated during osteogenic differentiation. In human and mouse prostate tumors, ectopic vascular calcification was predominately luminal and colocalized with the endothelial marker CD31. Thus, prostate tumor endothelial cells are atypically multipotent and can undergo a mesenchymal-like transition.
Figures

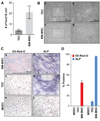
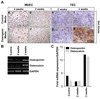
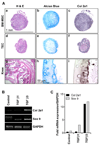
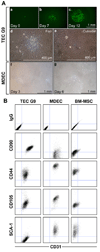
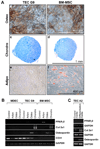
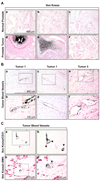
Comment in
-
Bony endothelium: tumor-mediated transdifferentiation?Cancer Cell. 2008 Sep 9;14(3):193-4. doi: 10.1016/j.ccr.2008.08.005. Cancer Cell. 2008. PMID: 18772107
References
-
- Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res. 2006;66:2173–2180. - PubMed
-
- Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102–111. - PubMed
-
- Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

