Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents
- PMID: 18772115
- PMCID: PMC2565587
- DOI: 10.1016/j.ccr.2008.08.001
Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents
Abstract
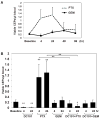
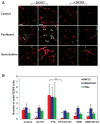
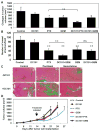
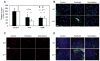
Several hypotheses have been proposed to explain how antiangiogenic drugs enhance the treatment efficacy of cytotoxic chemotherapy, including impairing the ability of chemotherapy-responsive tumors to regrow after therapy. With respect to the latter, we show that certain chemotherapy drugs, e.g., paclitaxel, can rapidly induce proangiogenic bone marrow-derived circulating endothelial progenitor (CEP) mobilization and subsequent tumor homing, whereas others, e.g., gemcitabine, do not. Acute CEP mobilization was mediated, at least in part, by systemic induction of SDF-1alpha and could be prevented by various procedures such as treatment with anti-VEGFR2 blocking antibodies or paclitaxel treatment in CEP-deficient Id mutant mice, both of which resulted in enhanced antitumor effects mediated by paclitaxel, but not by gemcitabine.
Figures


Comment in
-
Redefining the target again: chemotherapeutics as vascular disrupting agents?Cancer Cell. 2008 Sep 9;14(3):195-6. doi: 10.1016/j.ccr.2008.08.006. Cancer Cell. 2008. PMID: 18772108
References
-
- Addison CL, Arenberg DA, Morris SB, Xue YY, Burdick MD, Mulligan MS, Iannettoni MD, Strieter RM. The CXC chemokine, monokine induced by interferon-gamma, inhibits non-small cell lung carcinoma tumor growth and metastasis. Hum Gene Ther. 2000;11:247–261. - PubMed
-
- Athanassakis I, Papadimitriou L, Vassiliadis S. Murine ectoplacental cone-derived trophoblast cells express chemokine receptors. J Reprod Immunol. 2001;50:105–119. - PubMed
-
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, Hackett NR, Crystal RG, Witte L, Hicklin DJ, Bohlen P, Eaton D, Lyden D, de Sauvage F, Rafii S. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

