Disease-causing mutation in GPR54 reveals the importance of the second intracellular loop for class A G-protein-coupled receptor function
- PMID: 18772143
- PMCID: PMC2576551
- DOI: 10.1074/jbc.M805251200
Disease-causing mutation in GPR54 reveals the importance of the second intracellular loop for class A G-protein-coupled receptor function
Abstract
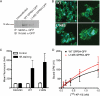
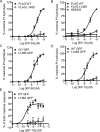
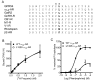
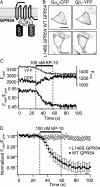
The G-protein-coupled receptor (GPCR) GPR54 is essential for the development and maintenance of reproductive function in mammals. A point mutation (L148S) in the second intracellular loop (IL2) of GPR54 causes idiopathic hypogonadotropic hypogonadism, a disorder characterized by delayed puberty and infertility. Here, we characterize the molecular mechanism by which the L148S mutation causes disease and address the role of IL2 in Class A GPCR function. Biochemical, immunocytochemical, and pharmacological analysis demonstrates that the mutation does not affect the expression, ligand binding properties, or protein interaction network of GPR54. In contrast, diverse GPR54 functional responses are markedly inhibited by the L148S mutation. Importantly, the leucine residue at this position is highly conserved among class A GPCRs. Indeed, mutating the corresponding leucine of the alpha(1A)-AR recapitulates the effects observed with L148S GPR54, suggesting the critical importance of this hydrophobic IL2 residue for Class A GPCR functional coupling. Interestingly, co-immunoprecipitation studies indicate that L148S does not hinder the association of Galpha subunits with GPR54. However, fluorescence resonance energy transfer analysis strongly suggests that L148S impairs the ligand-induced catalytic activation of Galpha. Combining our data with a predictive Class A GPCR/Galpha model suggests that IL2 domains contain a conserved hydrophobic motif that, upon agonist stimulation, might stabilize the switch II region of Galpha. Such an interaction could promote opening of switch II of Galpha to facilitate GDP-GTP exchange and coupling to downstream signaling responses. Importantly, mutations that disrupt this key hydrophobic interface can manifest as human disease.
Figures


References
-
- Tena-Sempere, M., and Huhtaniemi, I. T. (2003) in Reproductive Medicine: Molecular, Cellular and Genetic Fundamentals (Fauser, B., ed) pp. 225-244, Parthenon Publishing, New York
-
- Belchetz, P. E., Plant, T. M., Nakai, Y., Keogh, E. J., and Knobil, E. (1978) Science 202 631-633 - PubMed
-
- Seminara, S. B. (2006) Nat. Clin. Pract. Endocrinol. Metab. 2 328-334 - PubMed
-
- Iovane, A., Aumas, C., and de Roux, N. (2004) Eur. J. Endocrinol. 151 Suppl. 3, 83-88 - PubMed
-
- Bhagavath, B. M. D., and Layman, L. M. D. (2007) Semin. Reprod. Med. 25 272-286 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

