Differential origin of reticulospinal drive to motoneurons innervating trunk and hindlimb muscles in the mouse revealed by optical recording
- PMID: 18772205
- PMCID: PMC2652148
- DOI: 10.1113/jphysiol.2008.158105
Differential origin of reticulospinal drive to motoneurons innervating trunk and hindlimb muscles in the mouse revealed by optical recording
Abstract
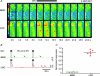
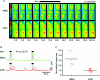
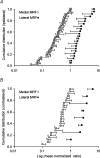
To better understand how the brainstem reticular formation controls and coordinates trunk and hindlimb muscle activity, we used optical recording to characterize the functional connections between medullary reticulospinal neurons and lumbar motoneurons of the L2 segment in the neonatal mouse. In an isolated brainstem-spinal cord preparation, synaptically induced calcium transients were visualized in individual MNs of the ipsilateral and contralateral medial and lateral motor columns (MMC, LMC) following focal electrical stimulation of the medullary reticular formation (MRF). Stimulation of the MRF elicited differential responses in MMC and LMC, according to a specific spatial organization. Stimulation of the medial MRF elicited responses predominantly in the LMC whereas stimulation of the lateral MRF elicited responses predominantly in the MMC. This reciprocal response pattern was observed on both the ipsilateral and contralateral sides of the spinal cord. To ascertain whether the regions stimulated contained reticulospinal neurons, we retrogradely labelled MRF neurons with axons coursing in different spinal funiculi, and compared the distributions of the labelled neurons to the stimulation sites. We found a large number of retrogradely labelled neurons within regions of the gigantocellularis reticular nucleus (including its pars ventralis and alpha) where most stimulation sites were located. The existence of a mediolateral organization within the MRF, whereby distinct populations of reticulospinal neurons predominantly influence medial or lateral motoneurons, provides an anatomical substrate for the differential control of trunk and hindlimb muscles. Such an organization introduces flexibility in the initiation and coordination of activity in the two sets of muscles that would satisfy many of the functional requirements that arise during postural and non-postural motor control in mammals.
Figures

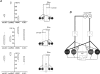


Similar articles
-
Organization of functional synaptic connections between medullary reticulospinal neurons and lumbar descending commissural interneurons in the neonatal mouse.J Neurosci. 2011 Mar 23;31(12):4731-42. doi: 10.1523/JNEUROSCI.5486-10.2011. J Neurosci. 2011. PMID: 21430172 Free PMC article.
-
Discharge properties of medullary reticulospinal neurons during postural changes induced by intrapontine injections of carbachol, atropine and serotonin, and their functional linkages to hindlimb motoneurons in cats.Exp Brain Res. 1994;99(3):361-74. doi: 10.1007/BF00228973. Exp Brain Res. 1994. PMID: 7957716
-
Imaging synaptically mediated responses produced by brainstem inputs onto identified spinal neurons in the neonatal mouse.J Neurosci Methods. 2009 May 30;180(1):1-8. doi: 10.1016/j.jneumeth.2009.01.018. Epub 2009 Jan 31. J Neurosci Methods. 2009. PMID: 19427523
-
Glutamatergic reticulospinal neurons in the mouse: developmental origins, axon projections, and functional connectivity.Ann N Y Acad Sci. 2013 Mar;1279:80-9. doi: 10.1111/nyas.12054. Ann N Y Acad Sci. 2013. PMID: 23531005 Review.
-
Vasopressin in the locus coeruleus and dorsal pontine tegmentum affects posture and vestibulospinal reflexes.Prog Brain Res. 1998;119:537-54. doi: 10.1016/s0079-6123(08)61592-7. Prog Brain Res. 1998. PMID: 10074811 Review.
Cited by
-
Organization of functional synaptic connections between medullary reticulospinal neurons and lumbar descending commissural interneurons in the neonatal mouse.J Neurosci. 2011 Mar 23;31(12):4731-42. doi: 10.1523/JNEUROSCI.5486-10.2011. J Neurosci. 2011. PMID: 21430172 Free PMC article.
-
Vestibular-mediated synaptic inputs and pathways to sympathetic preganglionic neurons in the neonatal mouse.J Physiol. 2012 Nov 15;590(22):5809-26. doi: 10.1113/jphysiol.2012.234609. Epub 2012 Sep 3. J Physiol. 2012. PMID: 22946097 Free PMC article.
-
Human Postural Control.Front Neurosci. 2018 Mar 20;12:171. doi: 10.3389/fnins.2018.00171. eCollection 2018. Front Neurosci. 2018. PMID: 29615859 Free PMC article. Review.
-
Lumbar V3 interneurons provide direct excitatory synaptic input onto thoracic sympathetic preganglionic neurons, linking locomotor, and autonomic spinal systems.Front Neural Circuits. 2023 Aug 28;17:1235181. doi: 10.3389/fncir.2023.1235181. eCollection 2023. Front Neural Circuits. 2023. PMID: 37701071 Free PMC article.
-
The diversity and plasticity of descending motor pathways rewired after stroke and trauma in rodents.Front Neural Circuits. 2025 Mar 21;19:1566562. doi: 10.3389/fncir.2025.1566562. eCollection 2025. Front Neural Circuits. 2025. PMID: 40191711 Free PMC article. Review.
References
-
- Alstermark B, Ogawa J. In vivo recordings of bulbospinal excitation in adult mouse forelimb motoneurons. J Neurophysiol. 2004;92:1958–1962. - PubMed
-
- Altman J, Bayer SA. Development of the brain stem in the rat. J Comp Neurol. 1980a;194:1–35. I. Thymidine-radiographic study of the time of origin of neurons of the lower medulla. - PubMed
-
- Altman J, Bayer SA. Development of the brain stem in the rat. J Comp Neurol. 1980b;194:37–56. II. Thymidine-radiographic study of the time of origin of neurons of the upper medulla, excluding the vestibular and auditory nuclei. - PubMed
-
- Auclair F, Bélanger MC, Marchand R. Ontogenetic study of early brain stem projections to the spinal cord in the rat. Brain Res Bull. 1993;30:281–389. - PubMed
-
- Auclair F, Marchand R, Glover JC. Regional patterning of reticulospinal and vestibulospinal neurons in the hindbrain of mouse and rat embryos. J Comp Neurol. 1999;411:288–300. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

