Progesterone influence on neurite outgrowth involves microglia
- PMID: 18772232
- PMCID: PMC2630906
- DOI: 10.1210/en.2008-0988
Progesterone influence on neurite outgrowth involves microglia
Abstract
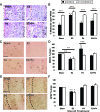
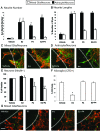
Progesterone (P4) antagonizes estradiol (E2) in synaptic remodeling in the hippocampus during the rat estrous cycle. To further understand how P4 modulates synaptic plasticity, we used entorhinal cortex lesions, which induce E2-dependent neurite sprouting in the hippocampus. In young ovariectomized rats, the E2-dependent entorhinal cortex lesion-induced sprouting was attenuated by concurrent treatment with P4 and E2. Microglial activation also showed the E2-P4 antagonism. These findings extend reports on the estrous cycle synaptic remodeling without lesions by showing the P4-E2 antagonism during simultaneous treatment with both E2 and P4. Glial mechanisms were analyzed with the wounding-in-a-dish model of cocultured glia and embryonic d-18 cortical neurons from rat. In cocultures of mixed glia (astrocytes plus 30% microglia), P4 antagonized the E2-dependent neurite outgrowth (number and length) and neuron viability in the presence of E2, as observed in vivo. However, removal of microglia (astrocyte-neuron coculture) abolished the antagonism of E2 by P4 on neuron sprouting. The P4 receptor antagonists ORG-31710 and RU-486 blocked the antagonism of P4 on E2-dependent sprouting. These findings suggest a new role for microglia in P4 antagonism of E2 in neuronal plasticity and show its dependence on progesterone receptors. These findings are also relevant to the inclusion of progestins in hormone therapy, which is controversial in relation to cognitive declines during aging and in Alzheimer's disease.
Figures

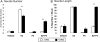
References
-
- Woolley CS, McEwen BS 1993 Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336:293–306 - PubMed
-
- Choi JM, Romeo RD, Brake WG, Bethea CL, Rosenwaks Z, McEwen BS 2003 Estradiol increases pre- and post-synaptic proteins in the CA1 regions of the hippocampus in female rhesus macaques (Macaca mulatta). Endocrinology 144:4734–4738 - PubMed
-
- McEwen BS 2002 Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol 91:2785–2801 - PubMed
-
- Chen E, Nilsen J, Brinton RD 2006 Dose and temporal pattern of estrogen exposure determines neuroprotective outcome in hippocampal neurons: therapeutic implications. Endocrinology 147:5303–5313 - PubMed

