Hypoxia and leucine deprivation induce human insulin-like growth factor binding protein-1 hyperphosphorylation and increase its biological activity
- PMID: 18772238
- PMCID: PMC2630895
- DOI: 10.1210/en.2008-0657
Hypoxia and leucine deprivation induce human insulin-like growth factor binding protein-1 hyperphosphorylation and increase its biological activity
Abstract
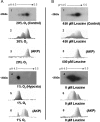
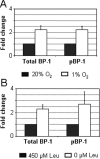
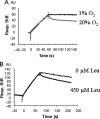
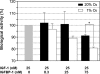
Fetal growth restriction is often caused by uteroplacental insufficiency that leads to fetal hypoxia and nutrient deprivation. Elevated IGF binding protein (IGFBP)-1 expression associated with fetal growth restriction has been documented. In this study we tested the hypothesis that hypoxia and nutrient deprivation induce IGFBP-1 phosphorylation and increase its biological potency in inhibiting IGF actions. HepG2 cells were subjected to hypoxia and leucine deprivation to mimic the deprivation of metabolic substrates. The total IGFBP-1 levels measured by ELISA were approximately 2- to 2.5-fold higher in hypoxia and leucine deprivation-treated cells compared with the controls. Two-dimensional immunoblotting showed that whereas the nonphosphorylated isoform is the predominant IGFBP-1 in the controls, the highly phosphorylated isoforms were dominant in hypoxia and leucine deprivation-treated cells. Liquid chromatography-tandem mass spectrometry analysis revealed four serine phosphorylation sites: three known sites (pSer 101, pSer 119, and pSer 169); and a novel site (pSer 98). Liquid chromatography-mass spectrometry was used to estimate the changes of phosphorylation upon treatment. Biacore analysis indicated that the highly phosphorylated IGFBP-1 isoforms found in hypoxia and leucine deprivation-treated cells had greater affinity for IGF-I [dissociation constant 5.83E (times 10 to the power)--0 m and 6.40E-09 m] relative to the IGFBP-1 from the controls (dissociation constant approximately 1.54E-07 m). Furthermore, the highly phosphorylated IGFBP-1 had a stronger effect in inhibiting IGF-I-stimulated cell proliferation. These findings suggest that IGFBP-1 phosphorylation may be a novel mechanism of fetal adaptive response to hypoxia and nutrient restriction.
Figures


Similar articles
-
Hypoxia Increases IGFBP-1 Phosphorylation Mediated by mTOR Inhibition.Mol Endocrinol. 2016 Feb;30(2):201-16. doi: 10.1210/me.2015-1194. Epub 2015 Dec 29. Mol Endocrinol. 2016. PMID: 26714229 Free PMC article.
-
Increased IGFBP-1 phosphorylation in response to leucine deprivation is mediated by CK2 and PKC.Mol Cell Endocrinol. 2016 Apr 15;425:48-60. doi: 10.1016/j.mce.2015.12.006. Epub 2015 Dec 28. Mol Cell Endocrinol. 2016. PMID: 26733150 Free PMC article.
-
Inhibition of decidual IGF-1 signaling in response to hypoxia and leucine deprivation is mediated by mTOR and AAR pathways and increased IGFBP-1 phosphorylation.Mol Cell Endocrinol. 2020 Jul 15;512:110865. doi: 10.1016/j.mce.2020.110865. Epub 2020 Jun 5. Mol Cell Endocrinol. 2020. PMID: 32502935
-
Phosphorylation of insulin-like growth factor binding proteins.Mol Cell Endocrinol. 1997 Apr 4;128(1-2):1-5. doi: 10.1016/s0303-7207(97)04032-x. Mol Cell Endocrinol. 1997. PMID: 9140069 Review.
-
Insulin-like growth factor binding protein-1: recent findings and new directions.Proc Soc Exp Biol Med. 1997 Dec;216(3):319-57. doi: 10.3181/00379727-216-44182. Proc Soc Exp Biol Med. 1997. PMID: 9402139 Review.
Cited by
-
Mechanisms linking hypoxia to phosphorylation of insulin-like growth factor binding protein-1 in baboon fetuses with intrauterine growth restriction and in cell culture.FASEB J. 2021 Sep;35(9):e21788. doi: 10.1096/fj.202100397R. FASEB J. 2021. PMID: 34425031 Free PMC article.
-
AMPK-mTORC1 pathway mediates hepatic IGFBP-1 phosphorylation in glucose deprivation: a potential molecular mechanism of hypoglycemia-induced impaired fetal growth.J Mol Endocrinol. 2024 Jan 31;72(3):e230137. doi: 10.1530/JME-23-0137. Print 2024 Apr 1. J Mol Endocrinol. 2024. PMID: 38194365 Free PMC article.
-
Children with idiopathic short stature have significantly different gut microbiota than their normal height siblings: a case-control study.Front Endocrinol (Lausanne). 2024 Feb 23;15:1343337. doi: 10.3389/fendo.2024.1343337. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38464968 Free PMC article.
-
Placental Remote Control of Fetal Metabolism: Trophoblast mTOR Signaling Regulates Liver IGFBP-1 Phosphorylation and IGF-1 Bioavailability.Int J Mol Sci. 2023 Apr 14;24(8):7273. doi: 10.3390/ijms24087273. Int J Mol Sci. 2023. PMID: 37108437 Free PMC article.
-
Hypoxia Increases IGFBP-1 Phosphorylation Mediated by mTOR Inhibition.Mol Endocrinol. 2016 Feb;30(2):201-16. doi: 10.1210/me.2015-1194. Epub 2015 Dec 29. Mol Endocrinol. 2016. PMID: 26714229 Free PMC article.
References
-
- Iwashita M 1994 [Physiological significance of IGF-I and its binding proteins on fetal growth and maturation]. Nippon Sanka Fujinka Gakkai Zasshi 46:660–672 (Japanese) - PubMed
-
- Martina NA, Kim E, Chitkara U, Wathen NC, Chard T, Giudice LC 1997 Gestational age-dependent expression of insulin-like growth factor-binding protein-1 (IGFBP-1) phosphoisoforms in human extraembryonic cavities, maternal serum, and decidua suggests decidua as the primary source of IGFBP-1 in these fluids during early pregnancy. J Clin Endocrinol Metab 82:1894–1898 - PubMed
-
- Sugawara J, Tazuke SI, Suen LF, Powell DR, Kaper F, Giaccia AJ, Giudice LC 2000 Regulation of insulin-like growth factor-binding protein 1 by hypoxia and 3′,5′-cyclic adenosine monophosphate is additive in HepG2 cells. J Clin Endocrinol Metab 85:3821–3827 - PubMed
-
- Ben Lagha N, Seurin D, Le Bouc Y, Binoux M, Berdal A, Menuelle P, Babajko S 2006 Insulin-like growth factor binding protein (IGFBP-1) involvement in intrauterine growth retardation: study on IGFBP-1 overexpressing transgenic mice. Endocrinology 147:4730–4737 - PubMed
-
- Watson CS, Bialek P, Anzo M, Khosravi J, Yee SP, Han VK 2006 Elevated circulating insulin-like growth factor binding protein-1 is sufficient to cause fetal growth restriction. Endocrinology 147:1175–1186 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

