Intra-amygdala injections of CREB antisense impair inhibitory avoidance memory: role of norepinephrine and acetylcholine
- PMID: 18772255
- PMCID: PMC2632786
- DOI: 10.1101/lm.904308
Intra-amygdala injections of CREB antisense impair inhibitory avoidance memory: role of norepinephrine and acetylcholine
Abstract
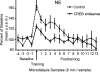
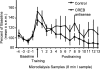
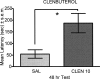
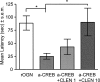
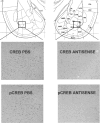
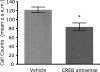
Infusions of CREB antisense into the amygdala prior to training impair memory for aversive tasks, suggesting that the antisense may interfere with CRE-mediated gene transcription and protein synthesis important for the formation of new memories within the amygdala. However, the amygdala also appears to modulate memory formation in distributed brain sites, through mechanisms that include the release of norepinephrine and acetylcholine within the amygdala. Thus, CREB antisense injections may affect memory by interfering with mechanisms of modulation, rather than storage, of memory. In the present experiment, rats received bilateral intra-amygdala infusions of CREB antisense (2 nmol/1 microL) 6 h prior to inhibitory avoidance training. In vivo microdialysis samples were collected from the right amygdala before, during, and following training. CREB antisense produced amnesia tested at 48 h after training. In addition, CREB antisense infusions dampened the training-related release of norepinephrine, and to a lesser extent of acetylcholine, in the amygdala. Furthermore, intra-amygdala infusions of the beta-adrenergic receptor agonist clenbuterol administered immediately after training attenuated memory impairments induced by intra-amygdala injections of CREB antisense. These findings suggest that intra-amygdala treatment with CREB antisense may affect processes involved in modulation of memory in part through interference with norepinephrine and acetylcholine neurotransmission in the amygdala.
Figures
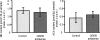

References
-
- Amaral D.G., Price J.L., Pitkänen A., Carmichael S.T. Anatomical organization of the primate amygdaloid complex. In: Aggleton J.P., editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. pp. 1–66.
-
- Aston-Jones G. Locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G., editor. The rat nervous system. Elsevier Academic Press; San Diego: 2004. pp. 259–294.
-
- Barco A., Pittenger C., Kandel E.R. CREB, memory enhancement and the treatment of memory disorders: Promises, pitfalls and prospects. Expert Opin. Ther. Targets. 2003;7:101–114. - PubMed
-
- Berlau D.J., McGaugh J.L. Enhancement of extinction memory consolidation: The role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol. Learn. Mem. 2006;86:123–132. - PubMed
-
- Bianchin M., Mello e Souza T., Medina J.H., Izquierdo I. The amygdala is involved in the modulation of long-term memory, but not in working or short-term memory. Neurobiol. Learn. Mem. 1999;71:127–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
