Progress in metabolic engineering of Saccharomyces cerevisiae
- PMID: 18772282
- PMCID: PMC2546860
- DOI: 10.1128/MMBR.00025-07
Progress in metabolic engineering of Saccharomyces cerevisiae
Abstract
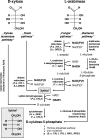
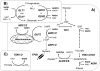
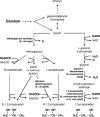
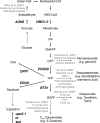
The traditional use of the yeast Saccharomyces cerevisiae in alcoholic fermentation has, over time, resulted in substantial accumulated knowledge concerning genetics, physiology, and biochemistry as well as genetic engineering and fermentation technologies. S. cerevisiae has become a platform organism for developing metabolic engineering strategies, methods, and tools. The current review discusses the relevance of several engineering strategies, such as rational and inverse metabolic engineering, evolutionary engineering, and global transcription machinery engineering, in yeast strain improvement. It also summarizes existing tools for fine-tuning and regulating enzyme activities and thus metabolic pathways. Recent examples of yeast metabolic engineering for food, beverage, and industrial biotechnology (bioethanol and bulk and fine chemicals) follow. S. cerevisiae currently enjoys increasing popularity as a production organism in industrial ("white") biotechnology due to its inherent tolerance of low pH values and high ethanol and inhibitor concentrations and its ability to grow anaerobically. Attention is paid to utilizing lignocellulosic biomass as a potential substrate.
Figures
References
-
- Abdel-Fattah, W. R., M. Fadil, P. Nigam, and I. M. Banat. 2000. Isolation of thermotolerant ethanologenic yeasts and use of selected strains in industrial scale fermentation in an Egyptian distillery. Biotechnol. Bioeng. 68531-535. - PubMed
-
- Acerenza, L. 2000. Design of large metabolic responses. Constraints and sensitivity analysis. J. Theor. Biol. 207265-282. - PubMed
-
- Aguilera, J., T. Petit, J. H. de Winde, and J. T. Pronk. 2005. Physiological and genome-wide transcriptional responses of Saccharomyces cerevisiae to high carbon dioxide concentrations. FEMS Yeast Res. 5579-593. - PubMed
-
- Akada, R. 2002. Genetically modified industrial yeast ready for application. J. Biosci. Bioeng. 94536-544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

