Convergence of molecular, modeling, and systems approaches for an understanding of the Escherichia coli heat shock response
- PMID: 18772288
- PMCID: PMC2546862
- DOI: 10.1128/MMBR.00007-08
Convergence of molecular, modeling, and systems approaches for an understanding of the Escherichia coli heat shock response
Abstract
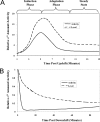
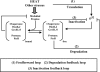
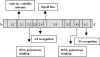
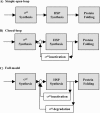
The heat shock response (HSR) is a homeostatic response that maintains the proper protein-folding environment in the cell. This response is universal, and many of its components are well conserved from bacteria to humans. In this review, we focus on the regulation of one of the most well-characterized HSRs, that of Escherichia coli. We show that even for this simple model organism, we still do not fully understand the central component of heat shock regulation, a chaperone-mediated negative feedback loop. In addition, we review other components that contribute to the regulation of the HSR in E. coli and discuss how these additional components contribute to regulation. Finally, we discuss recent genomic experiments that reveal additional functional aspects of the HSR.
Figures

References
-
- Bertani, D., A. B. Oppenheim, and F. Narberhaus. 2001. An internal region of the RpoH heat shock transcription factor is critical for rapid degradation by the FtsH protease. FEBS Lett. 49317-20. - PubMed
-
- Blaszczak, A., C. Georgopoulos, and K. Liberek. 1999. On the mechanism of FtsH-dependent degradation of the sigma 32 transcriptional regulator of Escherichia coli and the role of the Dnak chaperone machine. Mol. Microbiol. 31157-166. - PubMed
-
- Bugl, H., E. B. Fauman, B. L. Staker, F. Zheng, S. R. Kushner, M. A. Saper, J. C. Bardwell, and U. Jakob. 2000. RNA methylation under heat shock control. Mol. Cell 6349-360. - PubMed
-
- Chae, C., S. Sharma, J. R. Hoskins, and S. Wickner. 2004. CbpA, a DnaJ homolog, is a DnaK co-chaperone, and its activity is modulated by CbpM. J. Biol. Chem. 27933147-33153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

