IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function
- PMID: 18772313
- PMCID: PMC2936459
- DOI: 10.1634/stemcells.2008-0273
IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function
Abstract
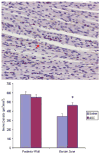
The administration of therapeutic cell types, such as stem and progenitor cells, has gained much interest for the limitation or repair of tissue damage caused by a variety of insults. However, it is still uncertain whether the morphological and functional benefits are mediated predominantly via cell differentiation or paracrine mechanisms. Here, we assessed the extent and mechanisms of adipose-derived stromal/stem cells (ASC)-dependent tissue repair in the context of acute myocardial infarction. Human ASCs in saline or saline alone was injected into the peri-infarct region in athymic rats following left anterior descending (LAD) coronary artery ligation. Cardiac function and structure were evaluated by serial echocardiography and histology. ASC-treated rats consistently exhibited better cardiac function, by all measures, than control rats 1 month following LAD occlusion. Left ventricular (LV) ejection fraction and fractional shortening were improved in the ASC group, whereas LV remodeling and dilation were limited in the ASC group compared with the saline control group. Anterior wall thinning was also attenuated by ASC treatment, and post-mortem histological analysis demonstrated reduced fibrosis in ASC-treated hearts, as well as increased peri-infarct density of both arterioles and nerve sprouts. Human ASCs were persistent at 1 month in the peri-infarct region, but they were not observed to exhibit significant cardiomyocyte differentiation. Human ASCs preserve heart function and augment local angiogenesis and cardiac nerve sprouting following myocardial infarction predominantly by the provision of beneficial trophic factors.
Conflict of interest statement
B.H.J. and K.L.M. have performed contract work for Lilly.
Figures





References
-
- Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. - PubMed
-
- Erbs S, Linke A, Schachinger V, et al. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: The Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation. 2007;116:366–374. - PubMed
-
- Lipinski MJ, Biondi-Zoccai GG, Abbate A, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. - PubMed
-
- Cao Y, Sun Z, Liao L, et al. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. - PubMed
-
- Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2004;109:656–663. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

