CX3CL1 up-regulation is associated with recruitment of CX3CR1+ mononuclear phagocytes and T lymphocytes in the lungs during cigarette smoke-induced emphysema
- PMID: 18772344
- PMCID: PMC2543064
- DOI: 10.2353/ajpath.2008.071034
CX3CL1 up-regulation is associated with recruitment of CX3CR1+ mononuclear phagocytes and T lymphocytes in the lungs during cigarette smoke-induced emphysema
Abstract
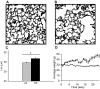
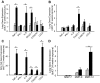
CX3CR1 is expressed on monocytes, dendritic cells, macrophages, subsets of T lymphocytes, and natural killer cells and functions in diverse capacities such as leukocyte adhesion, migration, and cell survival on ligand binding. Expression of the CX3CL1 gene, whose expression product is the sole ligand for CX3CR1, is up-regulated in human lungs with chronic cigarette smoke-induced obstructive lung disease. At present, it is unknown whether CX3CL1 up-regulation is associated with the recruitment and accumulation of immune cells that express CX3CR1. We show that mice chronically exposed to cigarette smoke up-regulate CX3CL1 gene expression, which is associated with an influx of CX3CR1+ cells in the lungs. The increase in CX3CR1+ cells is primarily comprised of macrophages and T lymphocytes and is associated with the development of emphysema. In alveolar macrophages, cigarette smoke exposure increased the expression of both CX3CR1 and CX3CL1 genes. The inducibility of CX3CR1 expression was not solely dependent on a chronic stimulus because lipopolysaccharide up-regulated CX3CR1 in RAW264.7 cells in vitro and in mononuclear phagocytes in vivo. Our findings suggest a mechanism by which macrophages amplify and promote CX3CR1+ cell accumulation within the lungs during both acute and chronic inflammatory stress. We suggest that one function of the CX3CR1-CX3CL1 pathway is to recruit and sustain divergent immune cell populations implicated in the pathogenesis of cigarette smoke-induced emphysema.
Figures





References
-
- MacKenzie TD, Bartecchi CE, Schrier RW. The human costs of tobacco use (2). N Engl J Med. 1994;330:975–980. - PubMed
-
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. - PubMed
-
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. - PubMed
-
- Shapiro SD, Ingenito EP. The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am J Respir Cell Mol Biol. 2005;32:367–372. - PubMed
-
- Finkelstein R, Fraser RS, Ghezzo H, Cosio MG. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med. 1995;152:1666–1672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

