NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes
- PMID: 18772377
- PMCID: PMC2563079
- DOI: 10.1073/pnas.0807328105
NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes
Abstract
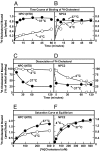
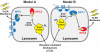
Egress of lipoprotein-derived cholesterol from lysosomes requires two lysosomal proteins, polytopic membrane-bound Niemann-Pick C1 (NPC1) and soluble Niemann-Pick C2 (NPC2). The reason for this dual requirement is unknown. Previously, we showed that the soluble luminal N-terminal domain (NTD) of NPC1 (amino acids 25-264) binds cholesterol. This NTD is designated NPC1(NTD). We and others showed that soluble NPC2 also binds cholesterol. Here, we establish an in vitro assay to measure transfer of [(3)H]cholesterol between these two proteins and phosphatidylcholine liposomes. Whereas NPC2 rapidly donates or accepts cholesterol from liposomes, NPC1(NTD) acts much more slowly. Bidirectional transfer of cholesterol between NPC1(NTD) and liposomes is accelerated >100-fold by NPC2. A naturally occurring human mutant of NPC2 (Pro120Ser) fails to bind cholesterol and fails to stimulate cholesterol transfer from NPC1(NTD) to liposomes. NPC2 may be essential to deliver or remove cholesterol from NPC1, an interaction that links both proteins to the cholesterol egress process from lysosomes. These findings may explain how mutations in either protein can produce a similar clinical phenotype.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
NPC1/NPC2 function as a tag team duo to mobilize cholesterol.Proc Natl Acad Sci U S A. 2008 Oct 7;105(40):15223-4. doi: 10.1073/pnas.0808256105. Epub 2008 Oct 1. Proc Natl Acad Sci U S A. 2008. PMID: 18832164 Free PMC article. No abstract available.
References
-
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. - PubMed
-
- Goldstein JL, Dana SE, Faust JR, Beaudet AL, Brown MS. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein: Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem. 1975;250:8487–8495. - PubMed
-
- Pentchev PG, Vanier MT, Suzuki K, Patterson MC. In: The Metabolic and Molecular Basis of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. New York: McGraw–Hill; 1995. pp. 2625–2639.
-
- Pentchev PG. Niemann–Pick C research from mouse to gene. Biochim Biophys Acta. 2004;1685:3–7. - PubMed
-
- Li AC, Tanaka RD, Callaway K, Fogelman AM, Edwards PA. Localization of 3-hydroxy-3-methylglutaryl CoA reductase and 3-hydroxy-3-methlyglutaryl CoA synthase in the rat liver and intestine is affected by cholestyramine and mevinolin. J Lipid Res. 1988;29:781–796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

