Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes
- PMID: 18772399
- PMCID: PMC2723798
- DOI: 10.1126/science.1161976
Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes
Abstract
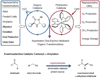
Photoredox catalysis and organocatalysis represent two powerful fields of molecule activation that have found widespread application in the areas of inorganic and organic chemistry, respectively. We merged these two catalysis fields to solve problems in asymmetric chemical synthesis. Specifically, the enantioselective intermolecular alpha-alkylation of aldehydes has been accomplished using an interwoven activation pathway that combines both the photoredox catalyst Ru(bpy)3Cl2 (where bpy is 2,2'-bipyridine) and an imidazolidinone organocatalyst. This broadly applicable, yet previously elusive, alkylation reaction is now highly enantioselective and operationally trivial.
Figures
Comment in
-
Chemistry. A light touch catalyzes asymmetric carbon-carbon bond formation.Science. 2008 Oct 3;322(5898):55-6. doi: 10.1126/science.1164403. Science. 2008. PMID: 18832635 No abstract available.
References
-
- Kalyanasundaram K. Coord. Chem. Rev. 1982;46:159.
-
- Juris A, et al. Coord. Chem. Rev. 1988;84:85.
-
- Renaud P, Sibi MP, editors. Radicals in Organic Synthesis. Weinheim, Germany: Wiley-VCH; 2001.
-
- Berkessel A, Gröger H, editors. Asymmetric Organocatalysis: From Biomimetic Concepts to Applications in Asymmetric Synthesis. Weinheim, Germany: Wiley-VCH; 2005.
-
- Dalko PI, editor. Enantioselective Organocatalysis: Reactions and Experimental Procedures. Weinheim, Germany: Wiley-VCH; 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources