The combined approach to lysis utilizing eptifibatide and rt-PA in acute ischemic stroke: the CLEAR stroke trial
- PMID: 18772447
- PMCID: PMC2588546
- DOI: 10.1161/STROKEAHA.108.517656
The combined approach to lysis utilizing eptifibatide and rt-PA in acute ischemic stroke: the CLEAR stroke trial
Abstract
Background and purpose: Multiple approaches are being studied to enhance the rate of thrombolysis for acute ischemic stroke. Treatment of myocardial infarction with a combination of a reduced-dose fibrinolytic agent and a glycoprotein (GP) IIb/IIIa receptor antagonist has been shown to improve the rate of recanalization versus fibrinolysis alone. The combined approach to lysis utilizing eptifibatide and recombinant tissue-type plasminogen activator (rt-PA) (CLEAR) stroke trial assessed the safety of treating acute ischemic stroke patients within 3 hours of symptom onset with this combination.
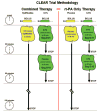
Methods: The CLEAR trial was a National Institutes of Health/National Institute of Neurological Disorders and Stroke-funded multicenter, double-blind, randomized, dose-escalation and safety study. Patients were randomized 3:1 to either low-dose rt-PA (tier 1=0.3 mg/kg, tier 2=0.45 mg/kg) plus eptifibatide (75 microg/kg bolus followed by 0.75 microg/kg per min infusion for 2 hours) or standard-dose rt-PA (0.9 mg/kg). The primary safety end point was the incidence of symptomatic intracerebral hemorrhage within 36 hours. Secondary analyses were performed regarding clinical efficacy.
Results: Ninety-four patients (40 in tier 1 and 54 in tier 2) were enrolled. The combination group of the 2 dose tiers (n=69) had a median age of 71 years and a median baseline National Institutes of Health Stroke Scale (NIHSS) score of 14, and the standard-dose rt-PA group (n=25) had a median age of 61 years and a median baseline NIHSS score of 10 (P=0.01 for NIHSS score). Fifty-two (75%) of the combination treatment group and 24 (96%) of the standard treatment group had a baseline modified Rankin scale score of 0 (P=0.04). There was 1 (1.4%; 95% CI, 0% to 4.3%) symptomatic intracranial hemorrhage in the combination group and 2 (8.0%; 95% CI, 0% to 19.2%) in the rt-PA-only arm (P=0.17). During randomization in tier 2, a review by the independent data safety monitoring board demonstrated that the safety profile of combination therapy at the tier 2 doses was such that further enrollment was statistically unlikely to indicate inadequate safety for the combination treatment group, the ultimate outcome of the study. Thus, the study was halted. There was a trend toward increased clinical efficacy of standard-dose rt-PA compared with the combination treatment group.
Conclusions: The safety of the combination of reduced-dose rt-PA plus eptifibatide justifies further dose-ranging trials in acute ischemic stroke.
Figures
References
-
- Tissue plasminogen activator for acute ischemic stroke: the National Institute of Neurological Disorders and Stroke rt-PA stroke study group. N Engl J Med. 1995;333:1581–1587. - PubMed
-
- Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59:862–867. - PubMed
-
- Pancioli AM, Brott TG. Therapeutic potential of platelet glycoprotein IIB/IIIA receptor antagonists in acute ischaemic stroke: scientific rationale and available evidence. CNS Drugs. 2004;18:981–988. - PubMed
-
- Inhibition of platelet glycoprotein IIB/IIIA with eptifibatide in patients with acute coronary syndromes: the PURSUIT trial investigators. Platelet glycoprotein IIB/IIIA in unstable angina: receptor suppression using integrilin therapy. N Engl J Med. 1998;339:436–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

