WASP confers selective advantage for specific hematopoietic cell populations and serves a unique role in marginal zone B-cell homeostasis and function
- PMID: 18772454
- PMCID: PMC2581999
- DOI: 10.1182/blood-2008-02-140715
WASP confers selective advantage for specific hematopoietic cell populations and serves a unique role in marginal zone B-cell homeostasis and function
Abstract
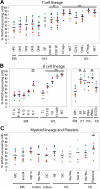
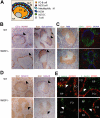
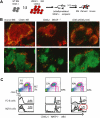
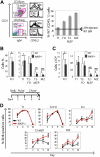
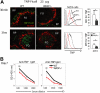
Development of hematopoietic cells depends on a dynamic actin cytoskeleton. Here we demonstrate that expression of the cytoskeletal regulator WASP, mutated in the Wiskott-Aldrich syndrome, provides selective advantage for the development of naturally occurring regulatory T cells, natural killer T cells, CD4(+) and CD8(+) T lymphocytes, marginal zone (MZ) B cells, MZ macrophages, and platelets. To define the relative contribution of MZ B cells and MZ macrophages for MZ development, we generated wild-type and WASP-deficient bone marrow chimeric mice, with full restoration of the MZ. However, even in the presence of MZ macrophages, only 10% of MZ B cells were of WASP-deficient origin. We show that WASP-deficient MZ B cells hyperproliferate in vivo and fail to respond to sphingosine-1-phosphate, a crucial chemoattractant for MZ B-cell positioning. Abnormalities of the MZ compartment in WASP(-/-) mice lead to aberrant uptake of Staphylococcus aureus and to a reduced immune response to TNP-Ficoll. Moreover, WASP-deficient mice have increased levels of "natural" IgM antibodies. Our findings reveal that WASP regulates both development and function of hematopoietic cells. We demonstrate that WASP deficiency leads to an aberrant MZ that may affect responses to blood-borne pathogens and peripheral B-cell tolerance.
Figures
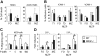
Comment in
-
WASp stings mature lymphocytes.Blood. 2008 Nov 15;112(10):3921-2. doi: 10.1182/blood-2008-09-176685. Blood. 2008. PMID: 18988873 Free PMC article.
References
-
- Notarangelo LD, Miao CH, Ochs HD. Wiskott-Aldrich syndrome. Curr Opin Hematol. 2008;15:30–36. - PubMed
-
- Filipovich AH, Stone JV, Tomany SC, et al. Impact of donor type on outcome of bone marrow transplantation for Wiskott-Aldrich syndrome: collaborative study of the International Bone Marrow Transplant Registry and the National Marrow Donor Program. Blood. 2001;97:1598–1603. - PubMed
-
- Pai SY, DeMartiis D, Forino C, et al. Stem cell transplantation for the Wiskott-Aldrich syndrome: a single-center experience confirms efficacy of matched unrelated donor transplantation. Bone Marrow Transplant. 2006;38:671–679. - PubMed
-
- Ozsahin H, Cavazzana-Calvo M, Notarangelo LD, et al. Long-term outcome following hematopoietic stem-cell transplantation in Wiskott-Aldrich syndrome: collaborative study of the European Society for Immunodeficiencies and European Group for Blood and Marrow Transplantation. Blood. 2008;111:439–445. - PubMed
-
- Dewey RA, Avedillo Diez I, Ballmaier M, et al. Retroviral WASP gene transfer into human hematopoietic stem cells reconstitutes the actin cytoskeleton in myeloid progeny cells differentiated in vitro. Exp Hematol. 2006;34:1161–1169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

