A comparison of the blood pressure changes of lumiracoxib with those of ibuprofen and naproxen
- PMID: 18772641
- PMCID: PMC8109916
- DOI: 10.1111/j.1751-7176.2008.07802.x
A comparison of the blood pressure changes of lumiracoxib with those of ibuprofen and naproxen
Abstract
The 52-week Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET) investigated the gastrointestinal and cardiovascular safety profile of lumiracoxib 400 mg once daily compared with 2 traditional nonsteroidal anti-inflammatory drugs (NSAIDs): ibuprofen 800 mg 3 times daily and naproxen 500 mg twice daily. Data from TARGET were analyzed to examine the effect of lumiracoxib compared with ibuprofen and naproxen on blood pressure (BP), incidence of de novo and aggravated hypertension, prespecified edema events, and congestive heart failure. Lumiracoxib resulted in smaller changes in BP as early as week 4. Least-squares mean change from baseline at week 4 for systolic BP was +0.57 mm Hg with lumiracoxib compared with +3.14 mm Hg with ibuprofen (P<.0001) and +0.43 with lumiracoxib compared with +1.80 mm Hg with naproxen (P<.0001). In conclusion, the use of lumiracoxib and traditional NSAIDs results in differing BP changes; these might be of clinical relevance.
Figures
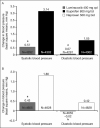
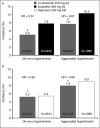
References
-
- Jeger RV, Greenberg JD, Ramanathan K, et al. Lumiracoxib, a highly selective COX‐2 inhibitor. Expert Review Clin Immunol. 2005;1:37–45. - PubMed
-
- Brater DC, Harris C, Redfern JS, et al. Renal effects of COX‐2‐selective inhibitors. Am J Nephrol. 2001;21:1–15. - PubMed
-
- Harris RC. Cyclooxygenase‐2 and the kidney: functional and pathophysiological implications. J Hypertens (Suppl). 2002;20(suppl 6):S3–S9. - PubMed
-
- Singh G, Miller JD, Huse DM, et al. Consequences of increased systolic blood pressure in patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 2003;30:714–719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

