Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats
- PMID: 18772869
- PMCID: PMC2597499
- DOI: 10.1038/nprot.2008.138
Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats
Abstract
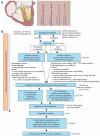
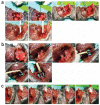
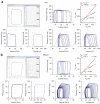
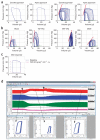
Ventricular pressure-volume relationships have become well established as the most rigorous and comprehensive ways to assess intact heart function. Thanks to advances in miniature sensor technology, this approach has been successfully translated to small rodents, allowing for detailed characterization of cardiovascular function in genetically engineered mice, testing effects of pharmacotherapies and studying disease conditions. This method is unique for providing measures of left ventricular (LV) performance that are more specific to the heart and less affected by vascular loading conditions. Here we present descriptions and movies for procedures employing this method (anesthesia, intubation and surgical techniques, calibrations). We also provide examples of hemodynamics measurements obtained from normal mice/rats, and from animals with cardiac hypertrophy/heart failure, and describe values for various useful load-dependent and load-independent indexes of LV function obtained using different types of anesthesia. The completion of the protocol takes 1-4 h (depending on the experimental design/end points).
Figures



References
-
- Sagawa K, Maughan WL, Suga H, Sunagawa K. Cardiac Contraction and the Pressure–Volume Relationship. Oxford University Press; New York: 1988.
-
- Kass DA, Yamazaki T, Burkhoff D, Maughan WL, Sagawa K. Determination of left ventricular end-systolic pressure–volume relationships by the conductance (volume) catheter technique. Circulation. 1986;73:586–595. - PubMed
-
- Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure–volume analysis: a guide for clinical, translational, and basic researchers. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H501–H512. - PubMed
-
- Little WC, Cheng CP. Effect of exercise on left ventricular-arterial coupling assessed in the pressure–volume plane. Am. J. Physiol. 1993;264:H1629–H1633. - PubMed
-
- Kass DA, Midei M, Graves W, Brinker JA, Maughan WL. Use of a conductance (volume) catheter and transient inferior vena caval occlusion for rapid determination of pressure–volume relationships in man. Cathet. Cardiovasc. Diagn. 1988;15:192–202. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

